
Bonding Molecular Orbital Two hydrogens,1s constructive overlap Constructive Interaction:The two Is orbitals are in phase and have the same sign add bonding molecular orbital represented by: => σ-bonding MO 6 Copyright2005 Pearson Prentice Hall,Inc
Chapter 2 6 Bonding Molecular Orbital Two hydrogens, 1s constructive overlap =>
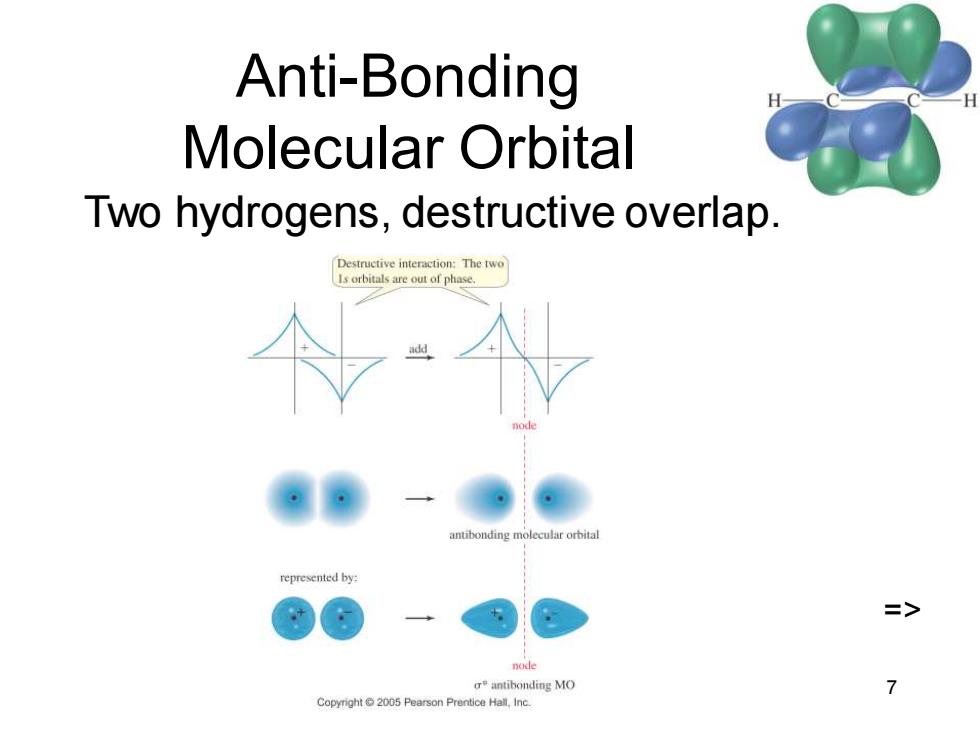
Anti-Bonding Molecular Orbital Two hydrogens,destructive overlap. Destructive interaction:The twe 1s orbitals are out of phase. anubonding molecular orbital represented by: => node antibonding MO 7 Pearson Prentice Hall,Inc
Chapter 2 7 Anti-Bonding Molecular Orbital Two hydrogens, destructive overlap. =>
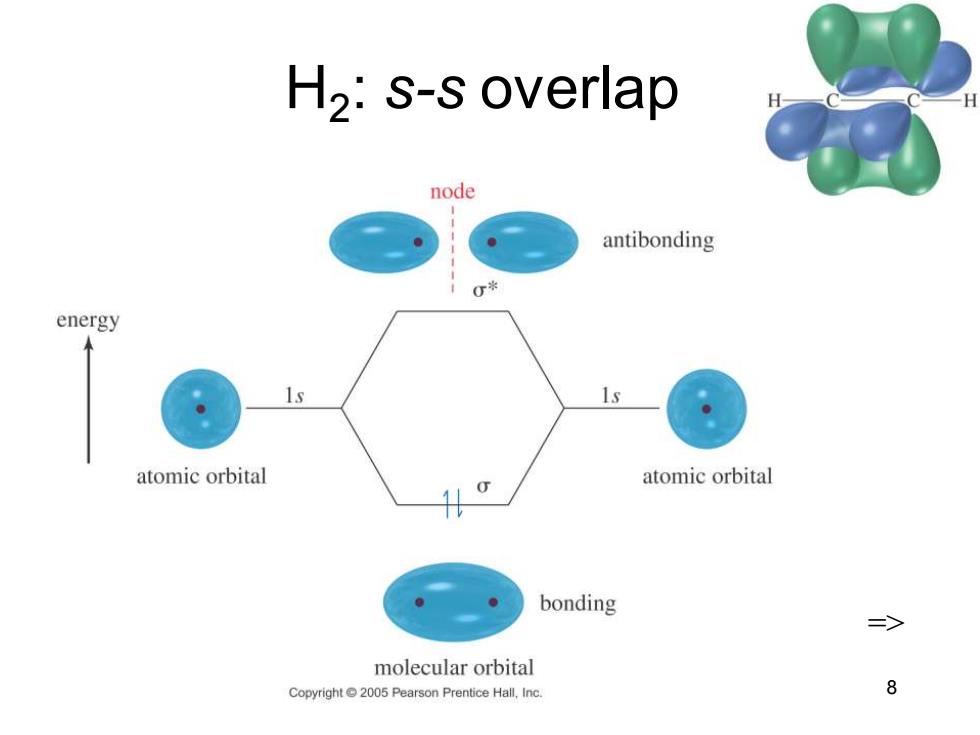
H2:s-s overlap node antibonding 0* energy atomic orbital atomic orbital bonding 三> molecular orbital Copyright 2005 Pearson Prentice Hall,Inc. 8
Chapter 2 8 H2 : s-s overlap =>
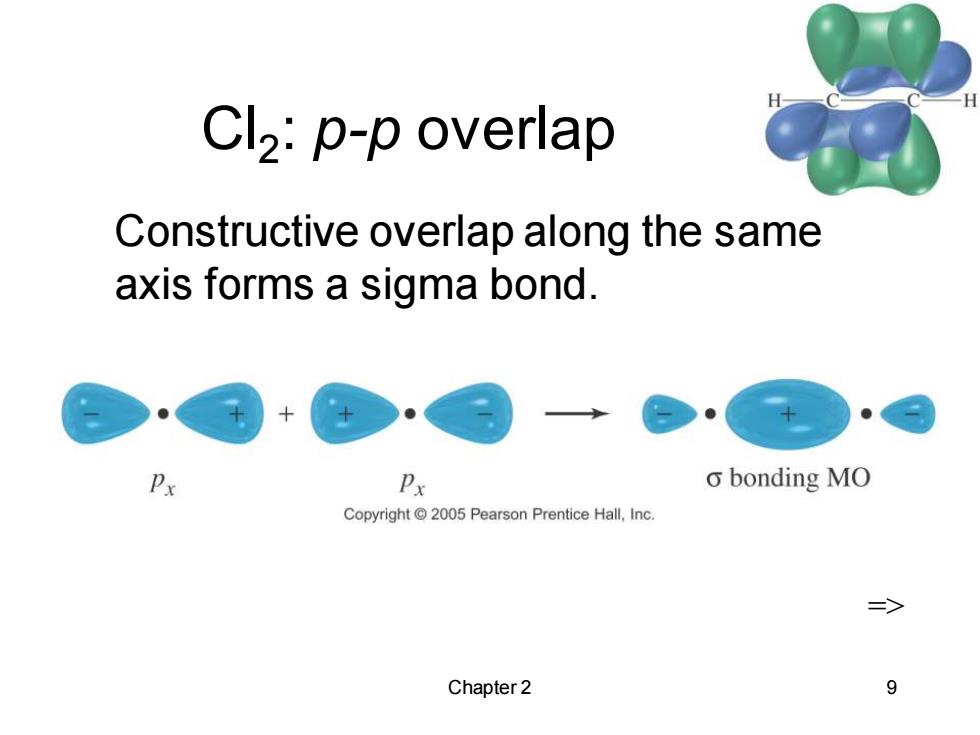
Cl2:p-p overlap Constructive overlap along the same axis forms a sigma bond. Px Px o bonding MO Copyright 2005 Pearson Prentice Hall,Inc. => Chapter 2 9
Chapter 2 9 Cl2 : p-p overlap => Constructive overlap along the same axis forms a sigma bond
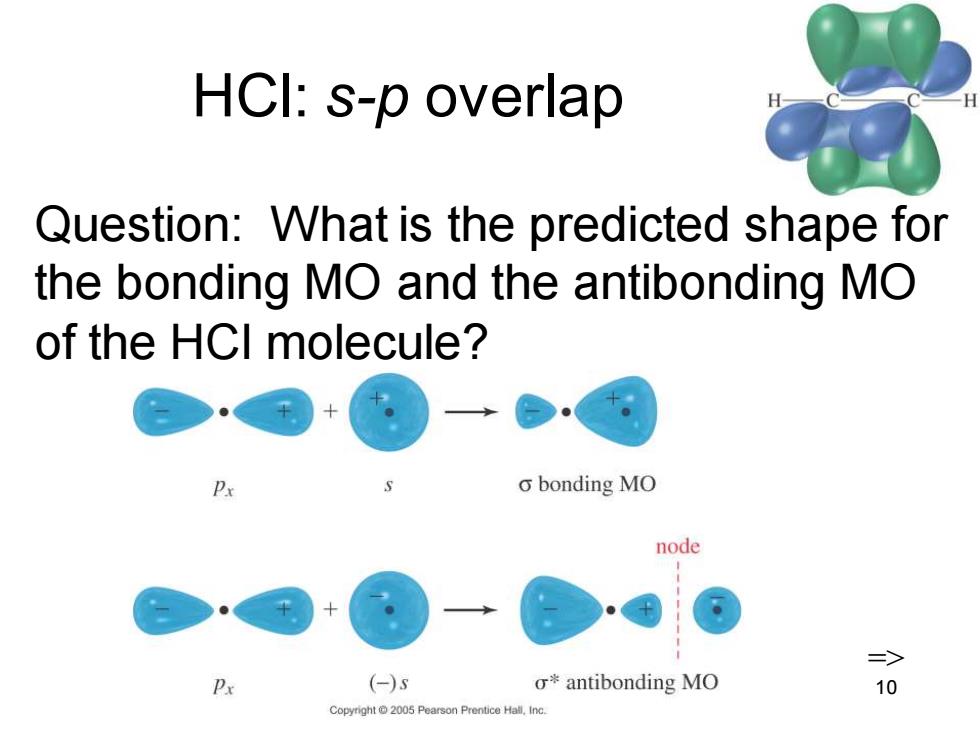
HCI:s-p overlap Question:What is the predicted shape for the bonding MO and the antibonding MO of the HCI molecule? o bonding MO node => Px (-)s o*antibonding MO 10 Copyright 2005 Pearson Prentice Hall.Inc
Chapter 2 10 HCl: s-p overlap Question: What is the predicted shape for the bonding MO and the antibonding MO of the HCl molecule? =>