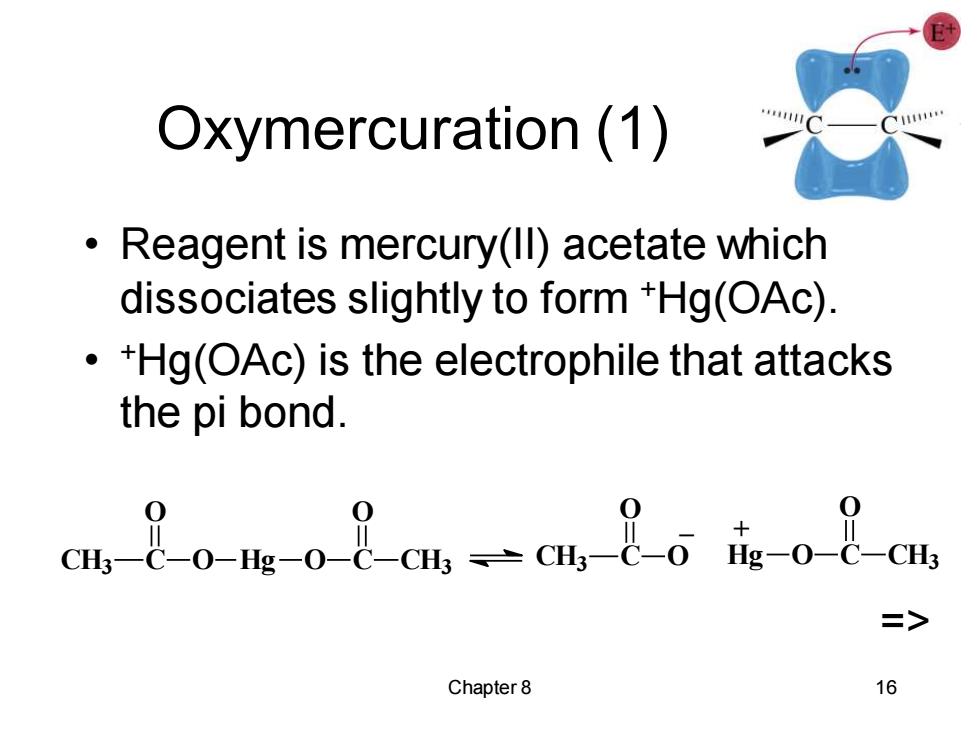
Oxymercuration(1) Reagent is mercury(ll)acetate which dissociates slightly to form *Hg(OAc) +Hg(OAc)is the electrophile that attacks the pi bond. Cm 8o-m-08cmc 8 Chapter 8 16
Chapter 8 16 Oxymercuration (1) • Reagent is mercury(II) acetate which dissociates slightly to form +Hg(OAc). • +Hg(OAc) is the electrophile that attacks the pi bond. CH3 C O O Hg O C O CH3 CH3 C O O _ Hg O C O CH3 + =>

Oxymercuration(2) The intermediate is a cyclic mercurinium ion,a three-membered ring with a positive charge. OAc He c-C Chapter 8
Chapter 8 17 Oxymercuration (2) The intermediate is a cyclic mercurinium ion, a three-membered ring with a positive charge. C C + Hg(OAc) C C Hg + OAc =>

Oxymercuration (3) Water approaches the mercurinium ion from the side opposite the ring (anti addition). Water adds to the more substituted carbon to form the Markovnikov product OAc OAc OAc Hg Hg H20: H 20 Chapter 8 18
Chapter 8 18 Oxymercuration (3) • Water approaches the mercurinium ion from the side opposite the ring (anti addition). • Water adds to the more substituted carbon to form the Markovnikov product. C C Hg + OAc H2O C O + C Hg H H OAc H2O C O C Hg H OAc =>

Demercuration Sodium borohydride,a reducing agent, replaces the mercury with hydrogen. OAc Hg NaBH +4 OH NaB(OH)4 4Hg +4 OAc H => Chapter 8 19
Chapter 8 19 Demercuration Sodium borohydride, a reducing agent, replaces the mercury with hydrogen. C O C Hg H OAc 4 4 C O C H H + NaBH4 + 4 OH _ + NaB(OH)4 + 4 Hg + 4 OAc _ =>

Predict the Product Predict the product when the given alkene reacts with aqueous mercuric acetate, followed by reduction with sodium borohydride. CH3 OH (1)Hg(OAc)2,H2O CH3 D (2)NaBH4 H anti addition Chapter 8 20
Chapter 8 20 Predict the Product Predict the product when the given alkene reacts with aqueous mercuric acetate, followed by reduction with sodium borohydride. CH3 D (1) Hg(OAc) 2, H2O (2) NaBH4 => OH CH3 D H anti addition