
Anti-Markovnikov ? C CH3-C-CH-CH3 CH3 Br CH3-C=CH-CH3 Br X CH3 CH-C-CH-CH Br Tertiary radical is more stable,so that intermediate forms faster. 二> Chapter8 11
Chapter 8 11 Anti-Markovnikov ?? • Tertiary radical is more stable, so that intermediate forms faster. => CH3 C CH3 CH CH3 + Br CH3 C CH3 CH CH3 Br CH3 C CH3 CH CH3 Br X
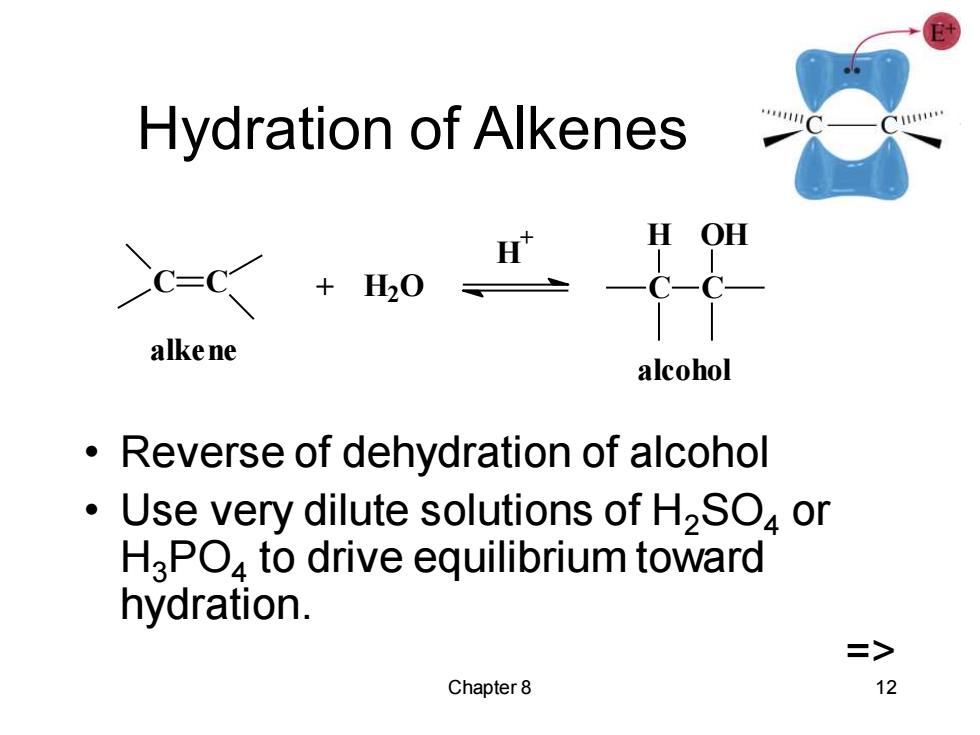
Hydration of Alkenes H OH alke ne alcohol Reverse of dehydration of alcohol Use very dilute solutions of H2SO4 or H3PO4 to drive equilibrium toward hydration. => Chapter 8 12
Chapter 8 12 Hydration of Alkenes • Reverse of dehydration of alcohol • Use very dilute solutions of H2SO4 or H3PO4 to drive equilibrium toward hydration. => C C + H2O H + C H C OH alkene alcohol
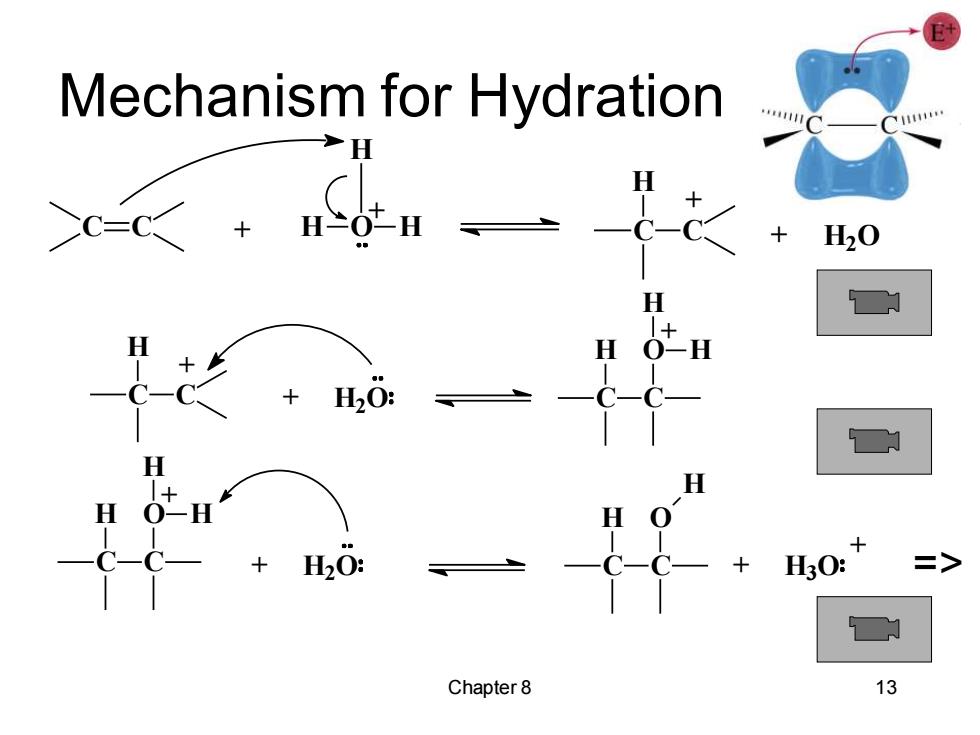
Mechanism for Hydration H H20 H HsO: Chapter 8 13
Chapter 8 13 Mechanism for Hydration C + H C + H2O C H C O H H + C + H2O H C O H H + C H C O H + H3O + => C C H O H H + + C + H2O H C +
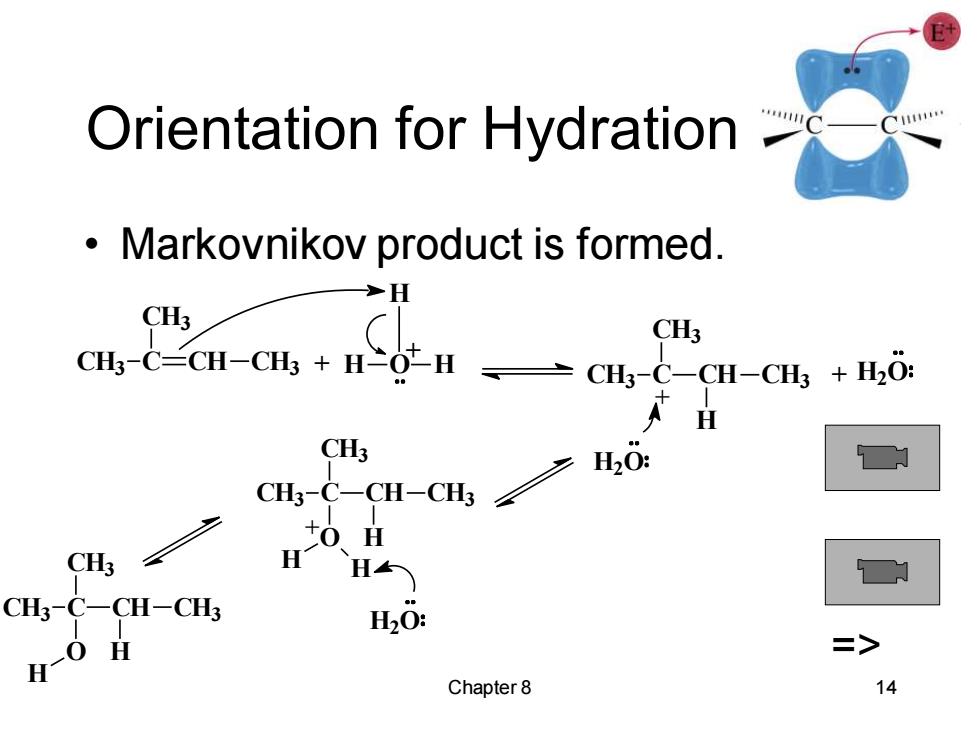
Orientation for Hydration 11 Markovnikov product is formed. H CH3 CH3 CH-C=CH-CH+H亡OH →CHC-CH-CH+,0: H C H20: 0.H H H CH3-C-CH-CH3 H20: H- Chapter 8 14
Chapter 8 14 Orientation for Hydration • Markovnikov product is formed. + CH3 C CH3 CH CH3 H O H H + + + H2O H CH CH3 CH3 CH3 C H2O CH3 C CH3 CH CH3 O H H H + H2O CH3 C CH3 CH CH3 O H H =>

Indirect Hydration Oxymercuration-Demercuration >Markovnikov product formed >Anti addition of H-OH >No rearrangements 。Hydroboration >Anti-Markovnikov product formed >Syn addition of H-OH => Chapter 8 15
Chapter 8 15 Indirect Hydration • Oxymercuration-Demercuration ➢Markovnikov product formed ➢Anti addition of H-OH ➢No rearrangements • Hydroboration ➢Anti-Markovnikov product formed ➢Syn addition of H-OH =>