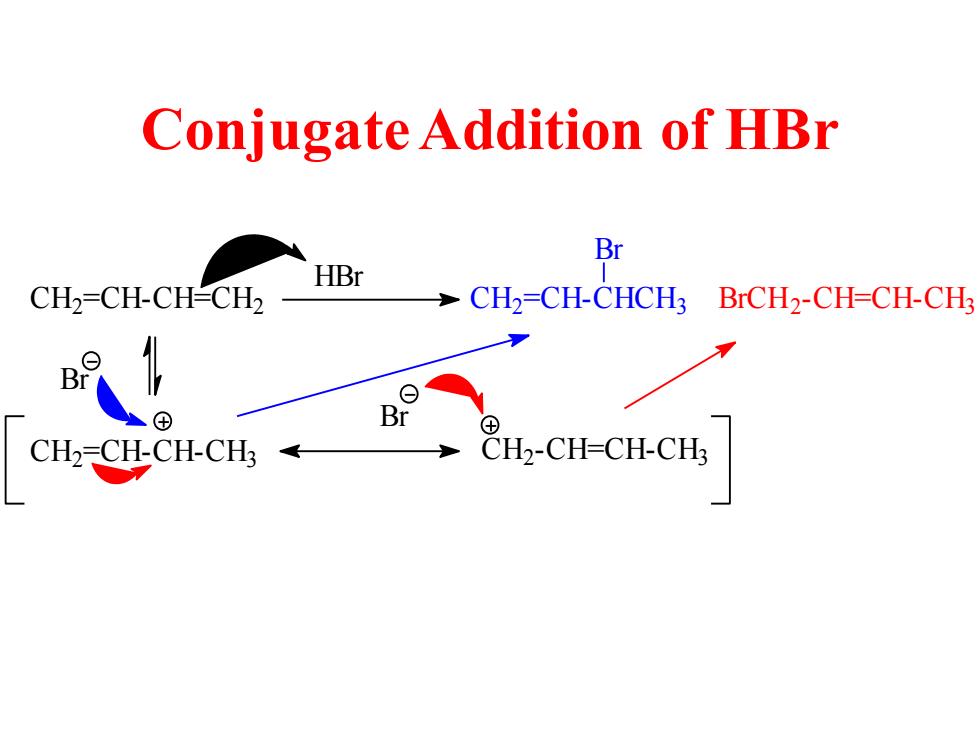
Conjugate Addition of HBr Br HBr CH2=CH-CH-CH2 CH2=CH-CHCH3 BrCH2-CH=CH-CH: + B ⊕ CH2-CH-CH-CH《 CH2-CH-CH-CH;
Conjugate Addition of HBr C H2 =CH-CH=CH2 HBr C H2 =CH-CH-CH3 C H2-CH=CH-CH3 C H2 =CH-CHCH3 Br BrCH2 -CH=CH-CH3 Br Br
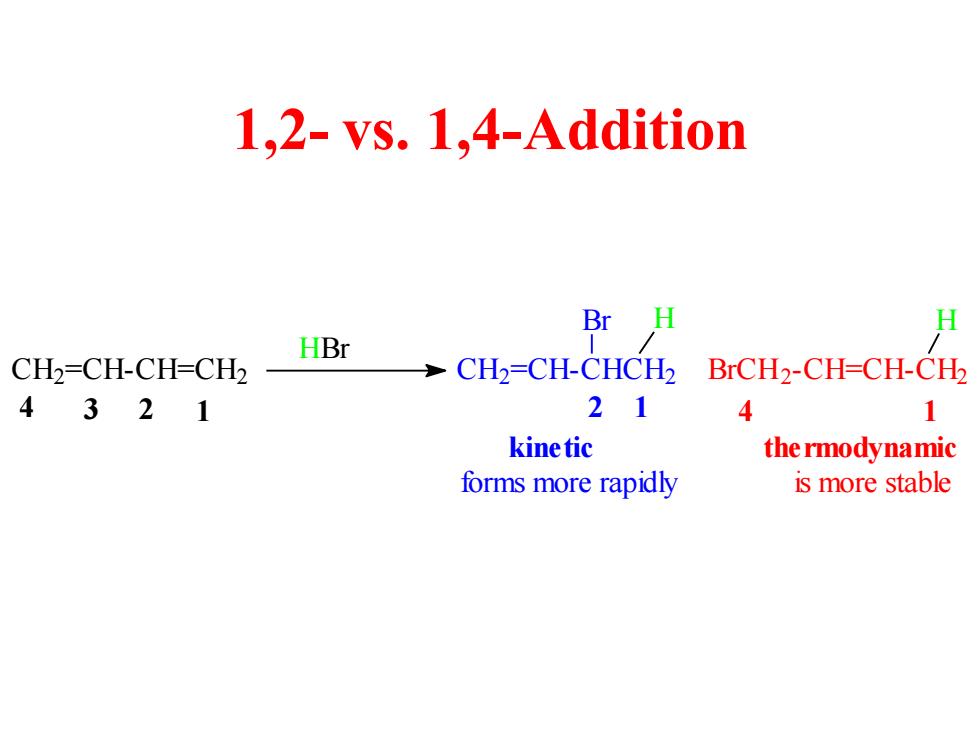
1,2-vs.1,4-Addition Br H HBr CH2=CH-CH-CH2 CH2=CH-CHCH2 BrCH2-CH=CH-CH2 4321 21 4 1 kine tic the rmodynamic forms more rapidly is more stable
1,2- vs. 1,4-Addition 4 3 2 1 2 1 4 1 H H kinetic thermodynamic C H2 =CH-CH=CH2 HBr C H2 =CH-CHCH2 Br BrCH2 -CH=CH-CH2 forms more rapidly is more stable
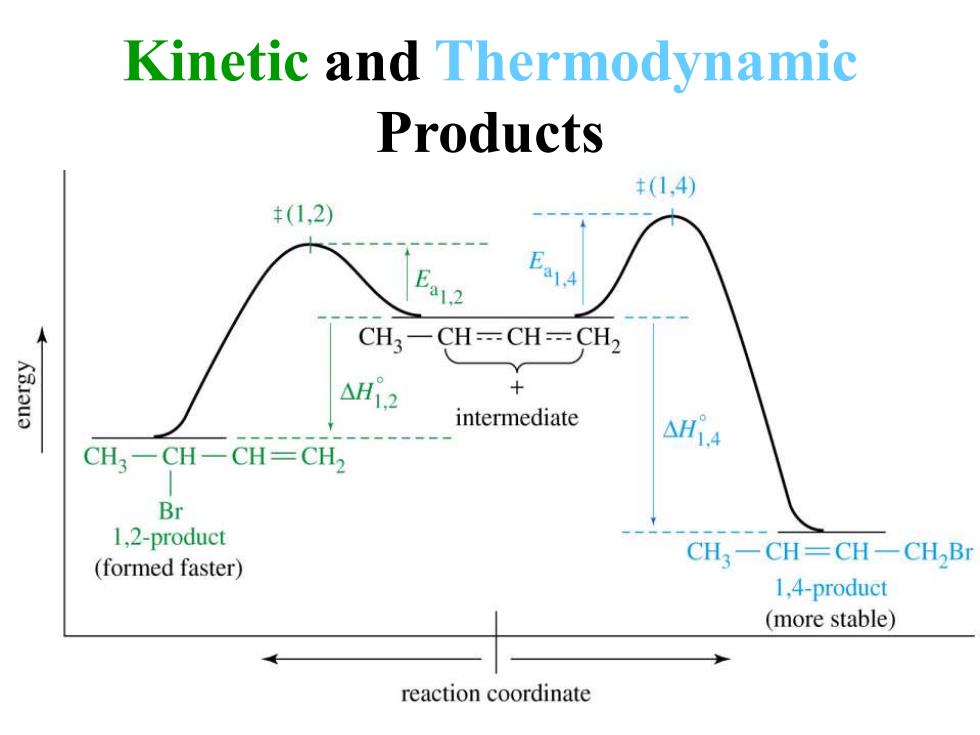
Kinetic and Thermodynamic Products (1,4) (1,2) E a1.2 CH3一CH=CH=CH2 KBJau3 △H2 + intermediate CH3-CH-CH-CH2 Br 1,2-product CH3一CH=CH-CHBI (formed faster) 1,4-product (more stable) reaction coordinate
Kinetic and Thermodynamic Products
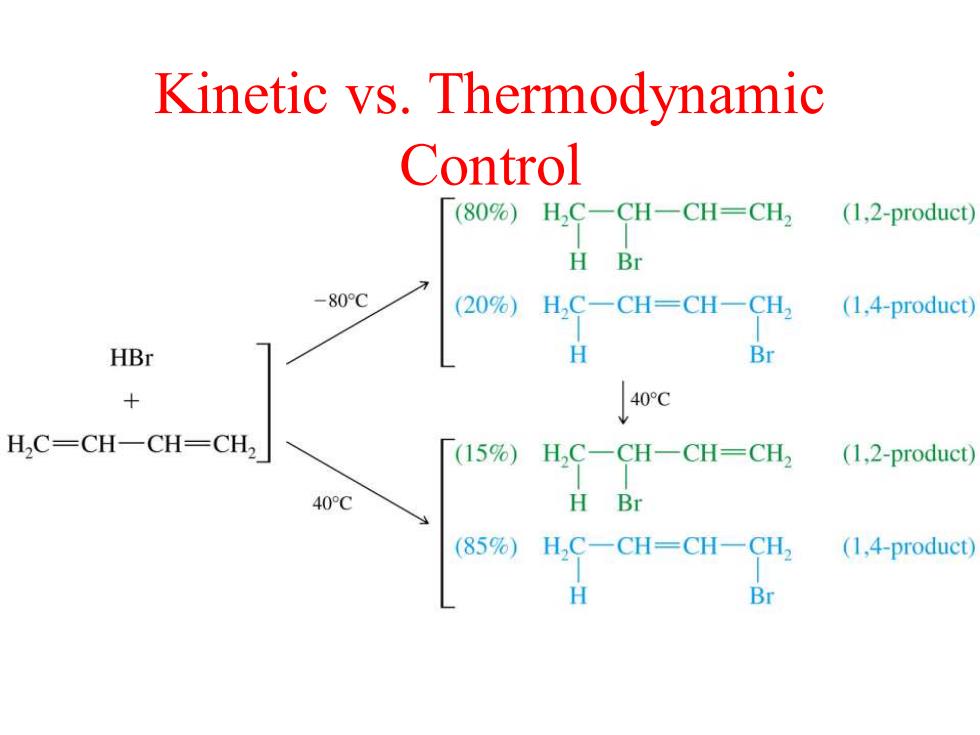
Kinetic vs.Thermodynamic Control (80%)H2C-CH-CH-CH2 (1,2-product) H Br -80C (20%)H,C一CH=CH一CH (1,4-product) HBr H Br × /40rc H2C=CH-CH=CH2 (15%) H,C-CH-CH=CH2 (1,2-product) 40C H Br (85%)H,C一CH=CH一CH2 (1,4-product) H Br
Kinetic vs. Thermodynamic Control
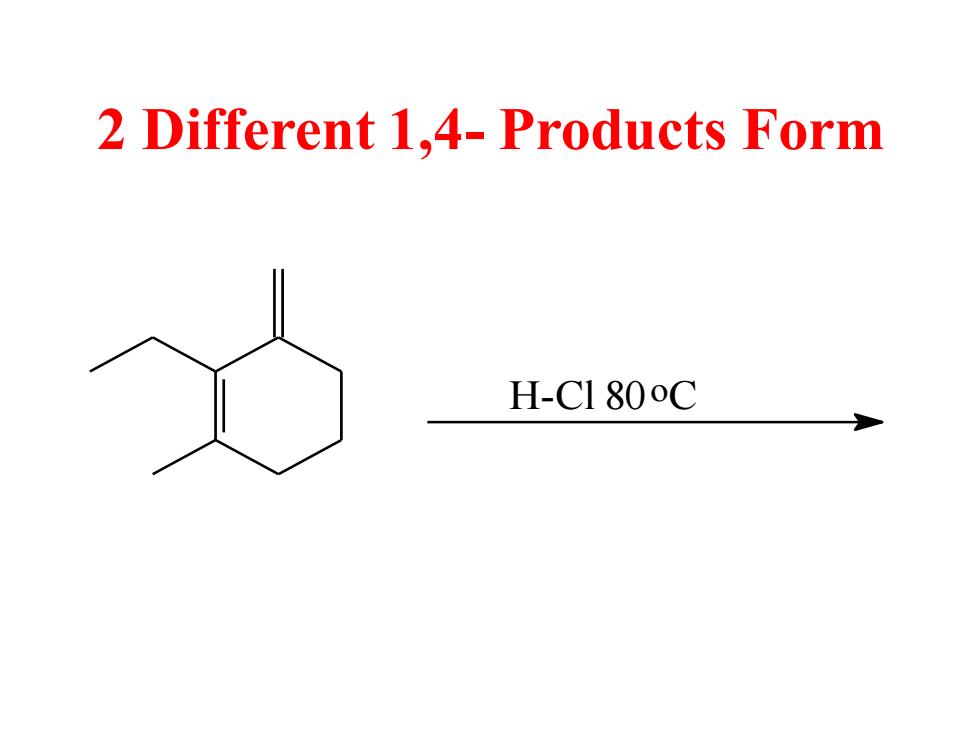
2 Different 1,4-Products Form H-C1 800C
2 Different 1,4- Products Form H-Cl 80o C