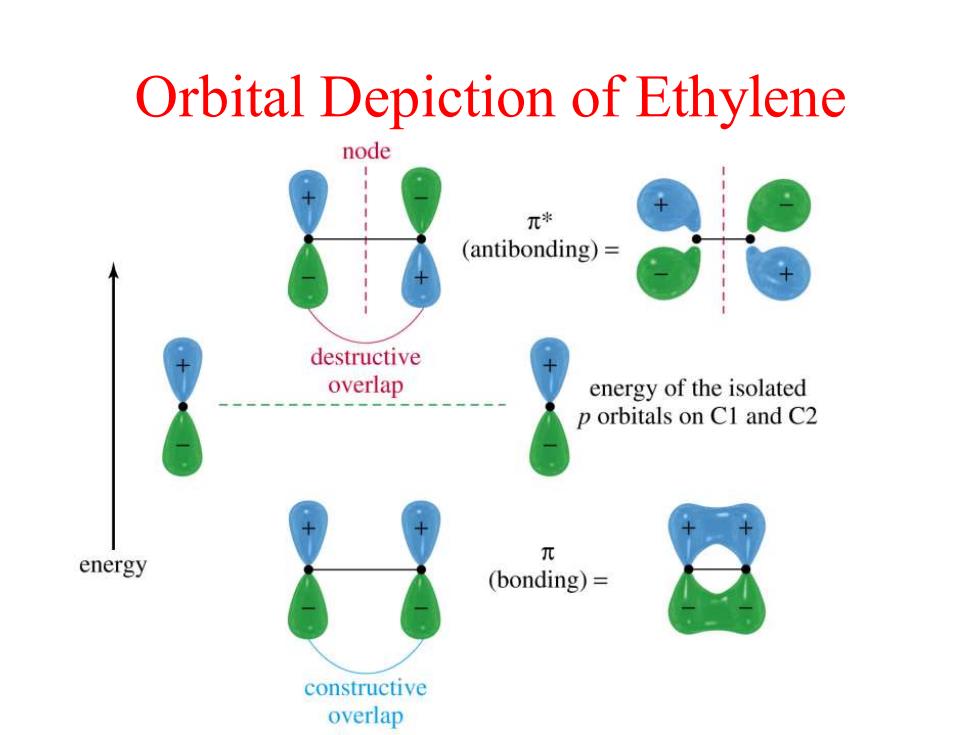
Orbital Depiction of Ethylene node π* (antibonding)= destructive overlap energy of the isolated p orbitals on C1 and C2 energy 元 (bonding)= constructive overlap
Orbital Depiction of Ethylene

Orbital Depiction small amount of overlap partial double bond H 1.34A H H H C 4H 1.48A 1.34A H H
Orbital Depiction
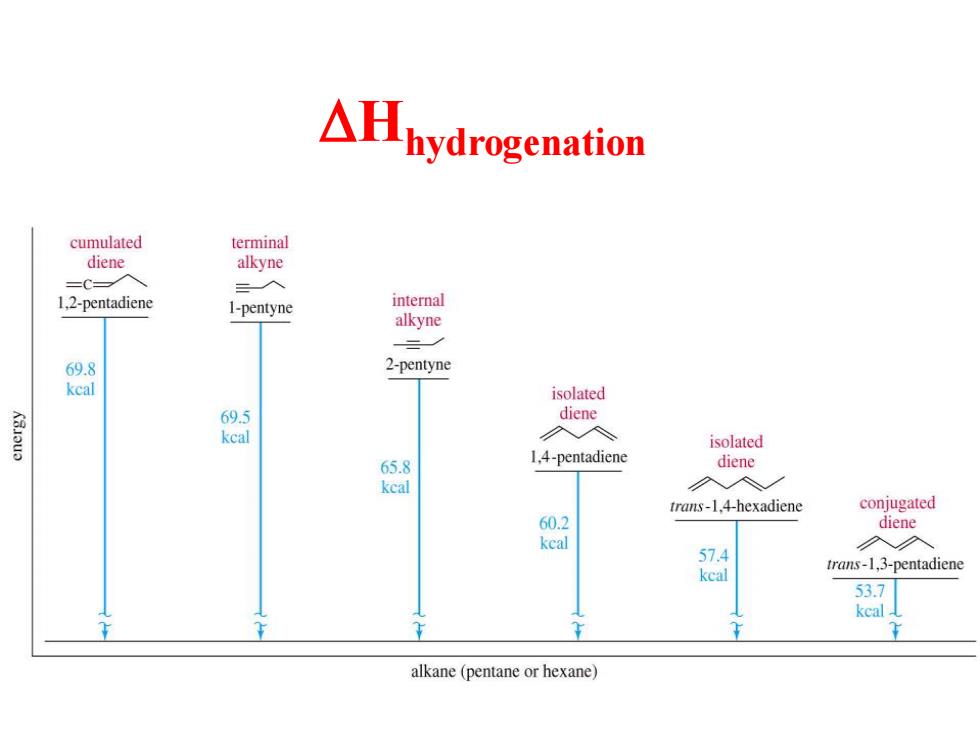
△hydrogenation cumulated terminal diene alkyne =C 1,2-pentadiene 1-pentyne internal alkyne 69.8 2-pentyne kcal isolated K3ou 69.5 diene kcal isolated 65.8 1,4-pentadiene diene kcal trans-1.4-hexadiene conjugated 60.2 diene keal 入◇ 57.4 kcal trans-1,3-pentadiene 53.7 kcal alkane (pentane or hexane)
DHhydrogenation
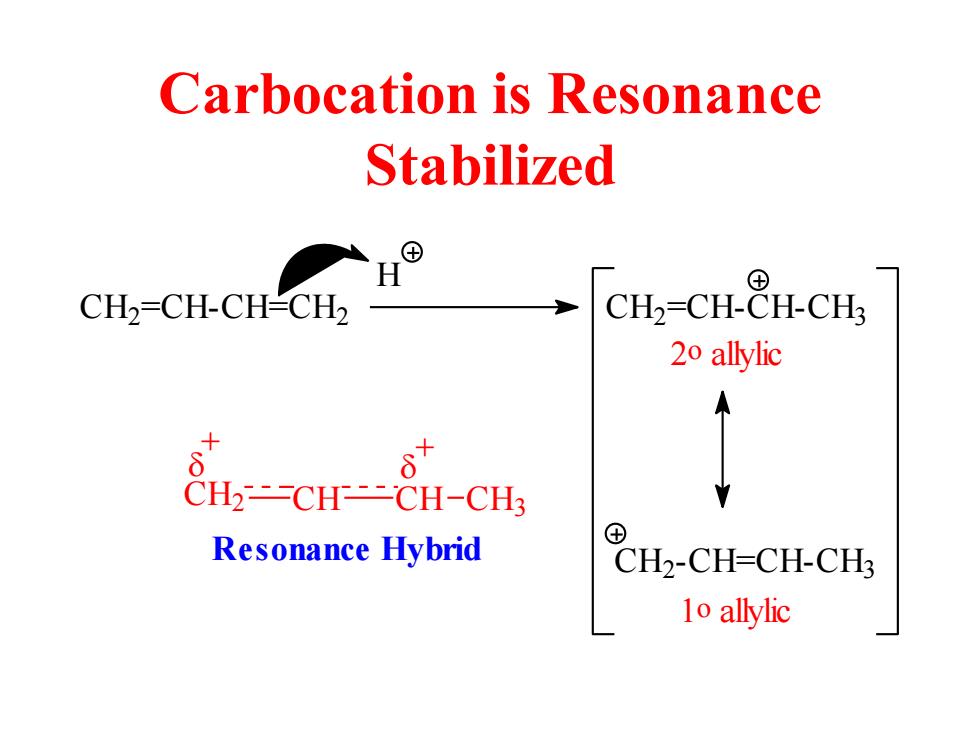
Carbocation is Resonance Stabilized ⊕ H ⊕ CH2=CH-CH-CH2 CH2-CH-CH-CH3 20 allylic CH2-CH-CH-CH3 Resonance Hybrid CH2-CH=CH-CH3 1o allylic
Carbocation is Resonance Stabilized C H2 =CH-CH=CH2 H C H2 =CH-CH-CH3 2o allylic C H2-CH=CH-CH3 1o allylic C H2 CH CH CH3 + + Resonance Hybrid
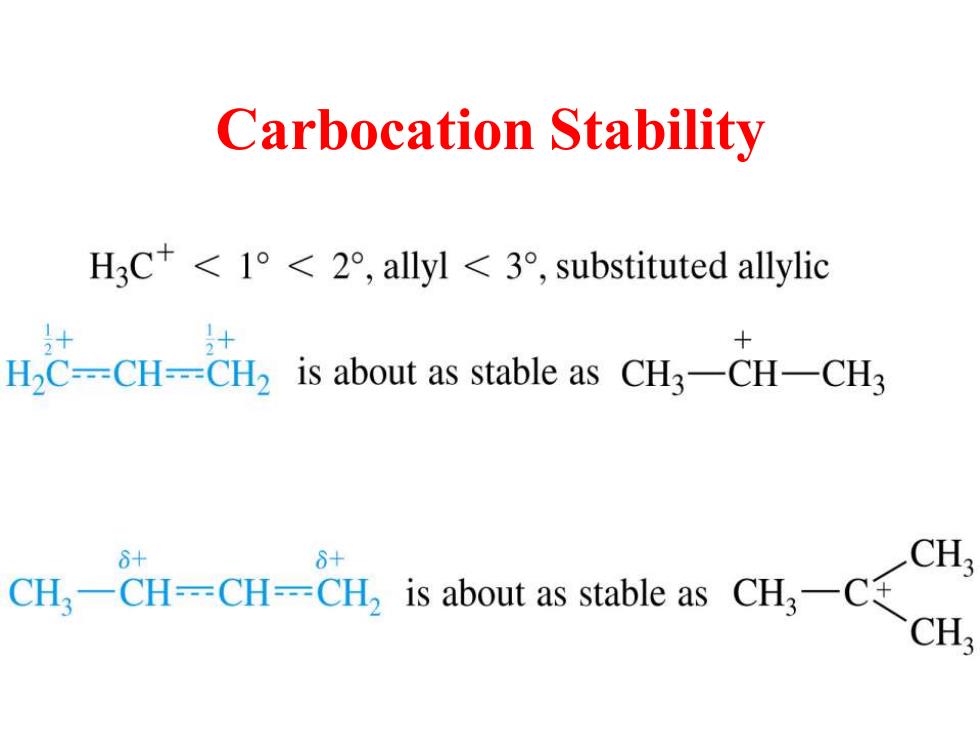
Carbocation Stability H3C+<1°<2°,allyl<3°,substituted allylic H2C--=CH---CH2 is about as stable as CH3-CH-CH3 8+ 6+ CH CH3-CH--CH--CH2 is about as stable as CH3-C+ CH
Carbocation Stability