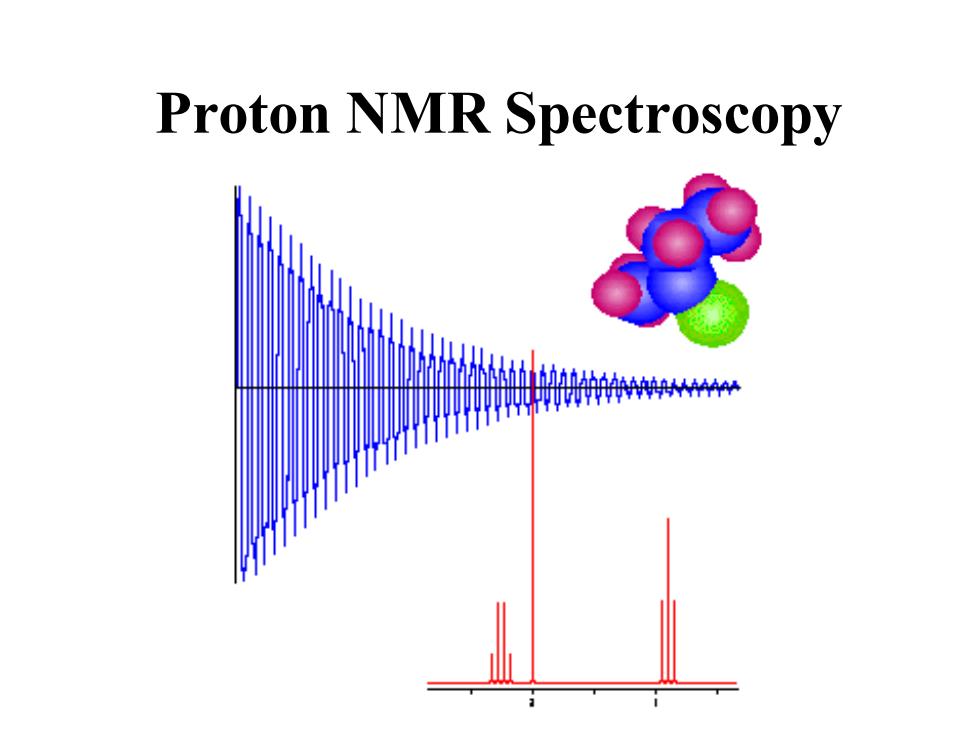
Proton NMR Spectroscopy
Proton NMR Spectroscopy
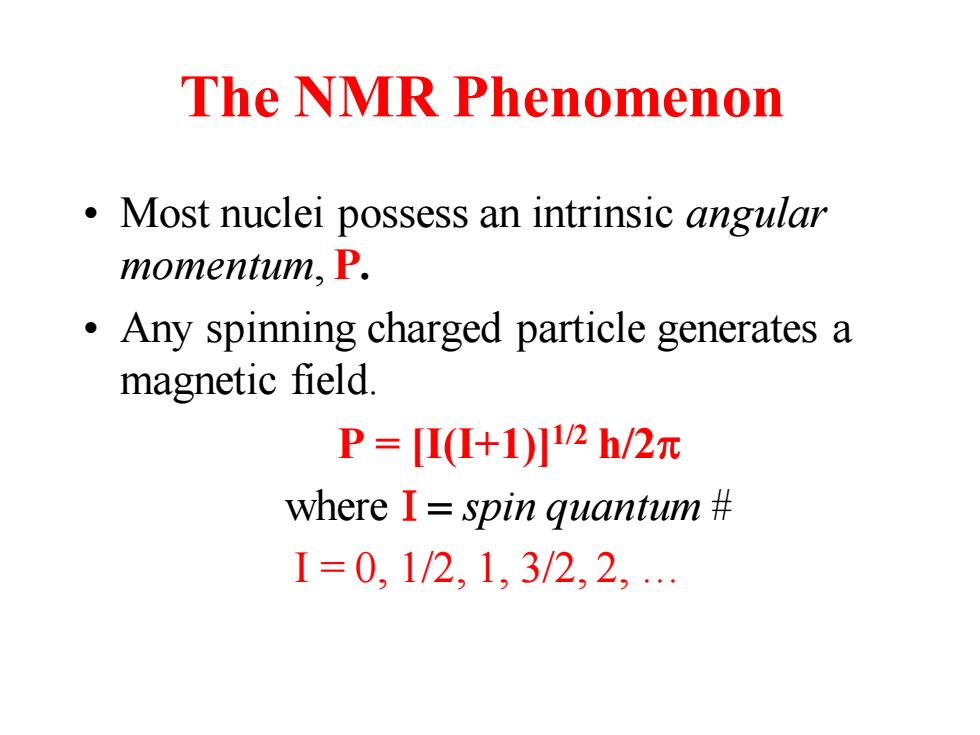
The NMR Phenomenon Most nuclei possess an intrinsic angular momentum,P. Any spinning charged particle generates a magnetic field. P=Π(+1)2h/2元 where I spin quantum I=0,1/2,1,3/2,2
The NMR Phenomenon • Most nuclei possess an intrinsic angular momentum, P. • Any spinning charged particle generates a magnetic field. P = [I(I+1)]1/2 h/2p where I = spin quantum # I = 0, 1/2, 1, 3/2, 2, …

Which nuclei have a "spin"? If mass and atomic are both even,I =0 and the nucleus has no spin. e.g.Carbon-12,Oxygen-16 For each nucleus with a spin,the of allowed spin states can be quantized: For a nucleus with I,there are 21+1 allowed spin states. H,13C,19F,31P all have I 1/2 △E=yh/2π)Bo
Which nuclei have a “spin”? • If mass # and atomic # are both even, I = 0 and the nucleus has no spin. e.g. Carbon-12, Oxygen-16 • For each nucleus with a spin, the # of allowed spin states can be quantized: • For a nucleus with I, there are 2I + 1 allowed spin states. 1H, 13C, 19F, 31P all have I = 1/2 DE = g(h/2p)Bo
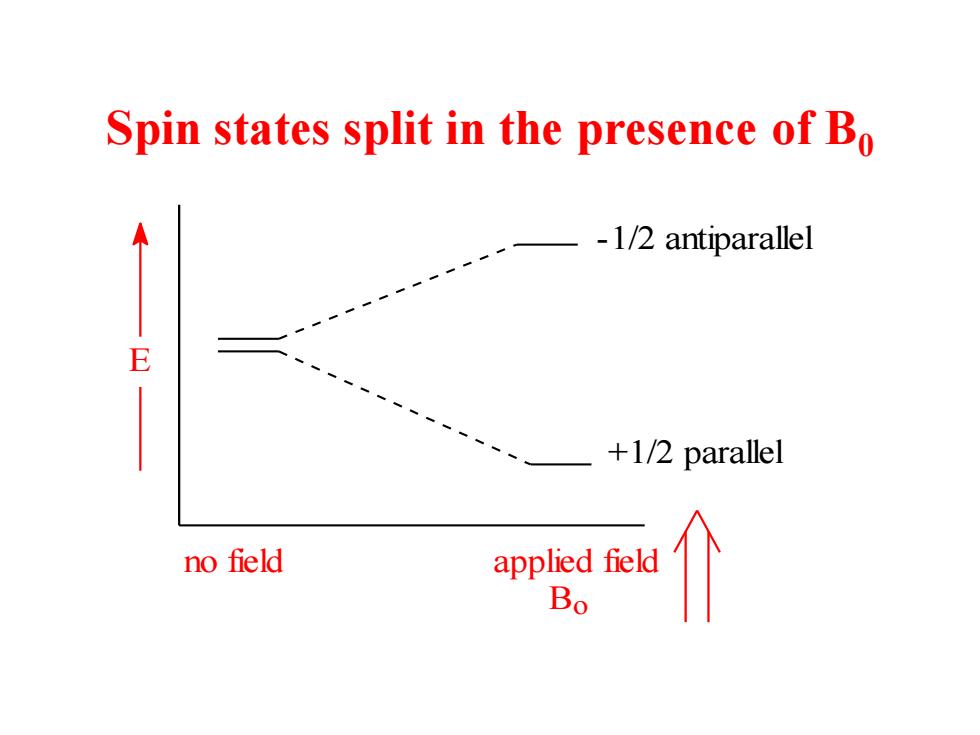
Spin states split in the presence of Bo -1/2 antiparallel E +1/2 parallel no field applied field Bo
Spin states split in the presence of B0 no field applied field E +1/2 parallel -1/2 antiparallel Bo
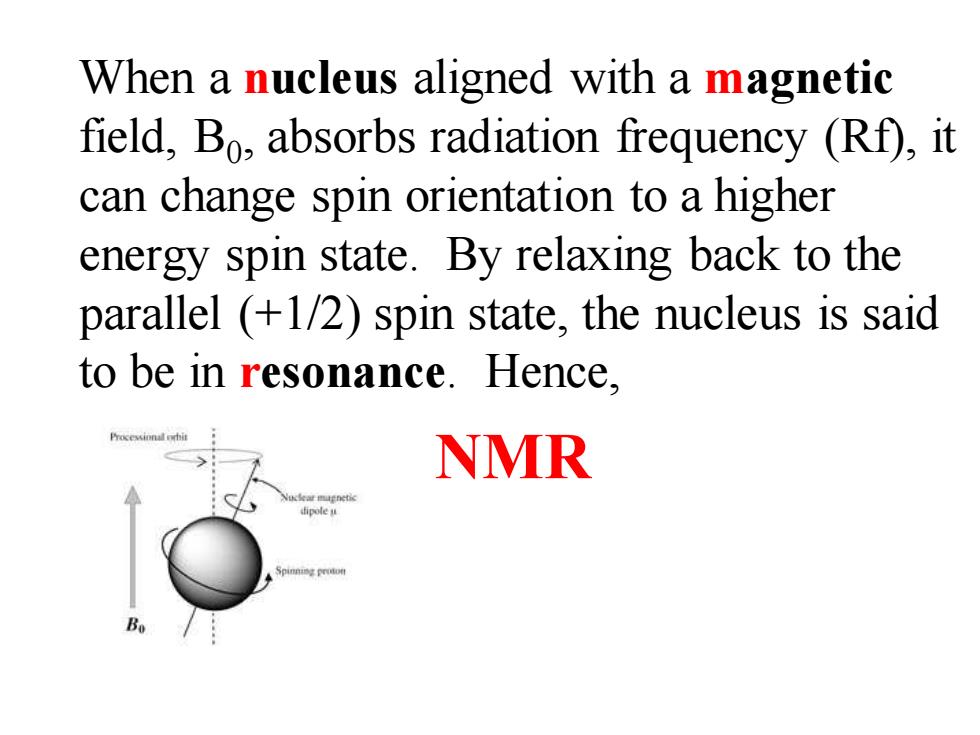
When a nucleus aligned with a magnetic field,Bo,absorbs radiation frequency (Rf),it can change spin orientation to a higher energy spin state.By relaxing back to the parallel (+1/2)spin state,the nucleus is said to be in resonance.Hence, NMR B
When a nucleus aligned with a magnetic field, B0 , absorbs radiation frequency (Rf), it can change spin orientation to a higher energy spin state. By relaxing back to the parallel (+1/2) spin state, the nucleus is said to be in resonance. Hence, NMR