
Section E Nucleophiles and Electrophiles Definition:Nucleophiles and electrophiles Active Intermediates: Anions Cations ;Charged Species; ■Radicals Neutral inorganic species
College of Science Section E Nucleophiles and Electrophiles Definition: Nucleophiles and electrophiles Active Intermediates: zAnions ; Cations ;Charged Species; Radicals Neutral inorganic species
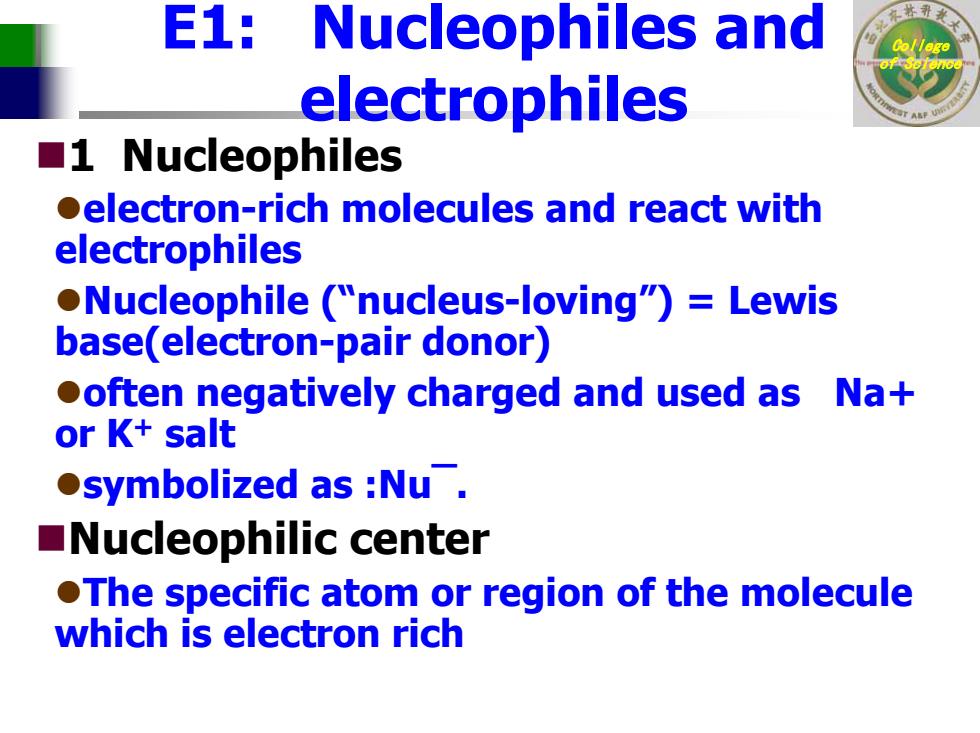
E1:Nucleophiles s and electrophiles ■1 Nucleophiles electron-rich molecules and react with electrophiles Nucleophile ("nucleus-loving")=Lewis base(electron-pair donor) ooften negatively charged and used as Na+ or K+salt ●symbolized as:Nu. ■Nucleophilic center OThe specific atom or region of the molecule which is electron rich
College of Science E1: Nucleophiles and electrophiles 1 Nucleophiles zelectron-rich molecules and react with electrophiles zNucleophile (“nucleus-loving”) = Lewis base(electron-pair donor) zoften negatively charged and used as Na+ or K + salt zsymbolized as :Nu¯. Nucleophilic center zThe specific atom or region of the molecule which is electron rich
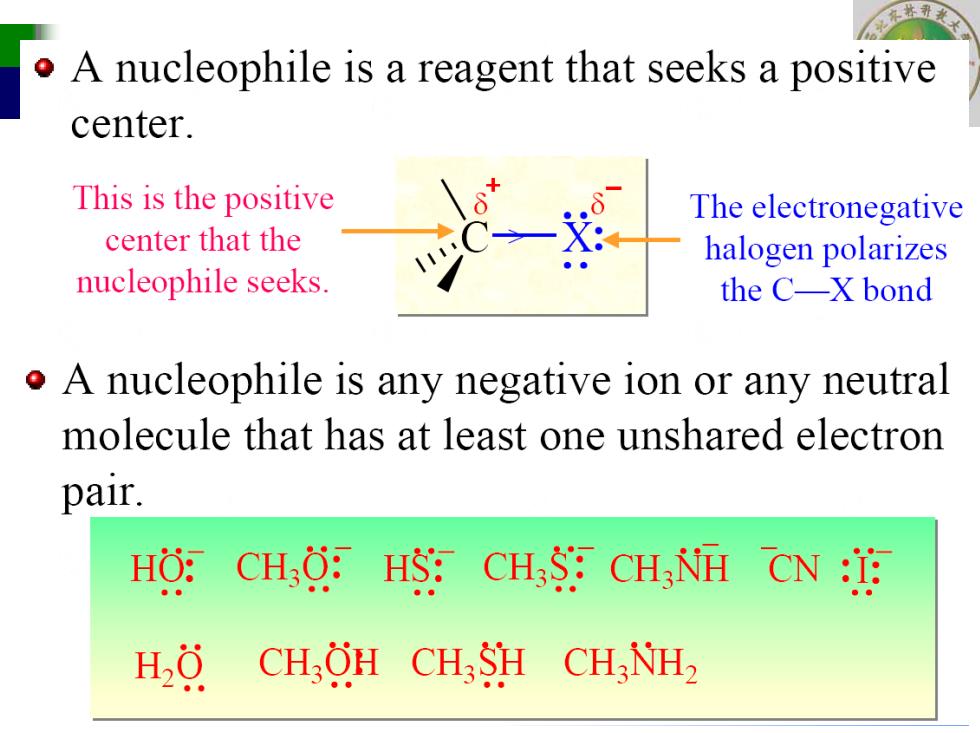
o A nucleophile is a reagent that seeks a positive center. This is the positive The electronegative center that the halogen polarizes nucleophile seeks. the C-X bond o A nucleophile is any negative ion or any neutral molecule that has at least one unshared electron pair. HO: CHO:HS:CH3S CH3NH CN :I H,0 CH,OH CH3SH CH3NH2
College of Science
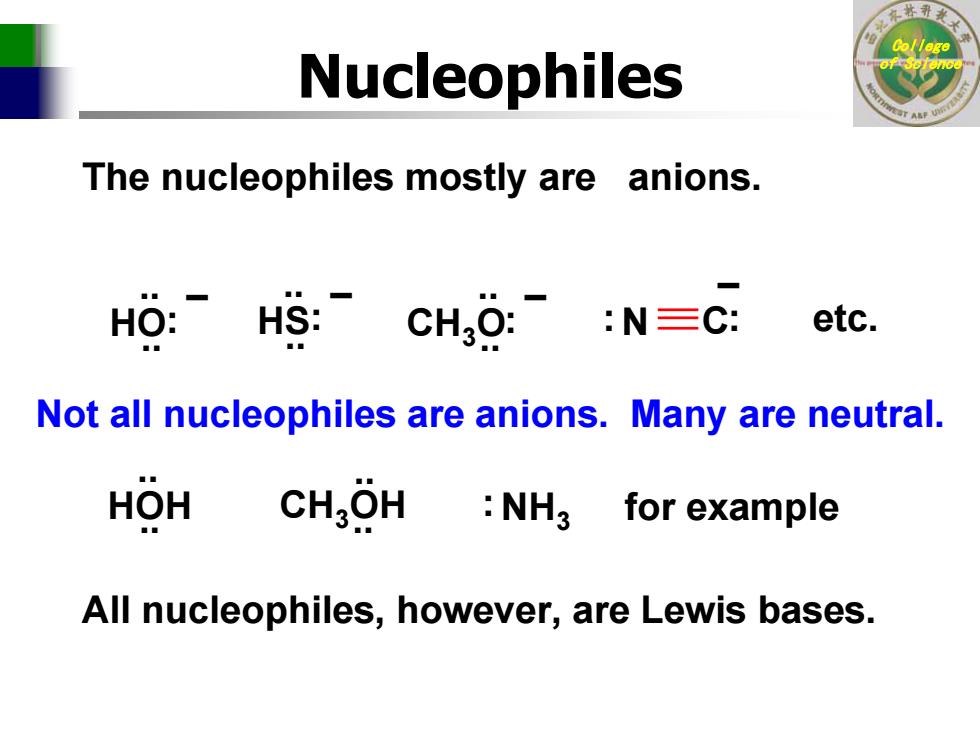
Nucleophiles The nucleophiles mostly are anions. HO:HS:: :N三C: etc. Not all nucleophiles are anions.Many are neutral. HOH CHgOH NH3 for example All nucleophiles,however,are Lewis bases
College of Science Nucleophiles The nucleophiles mostly are anions. – : : N C .. .. HS: .. – .. HO: – .. .. CH 3 O: – etc. Not all nucleophiles are anions. Many are neutral. .. .. HOH CH 3OH.. .. NH 3 : for example All nucleophiles, however, are Lewis bases
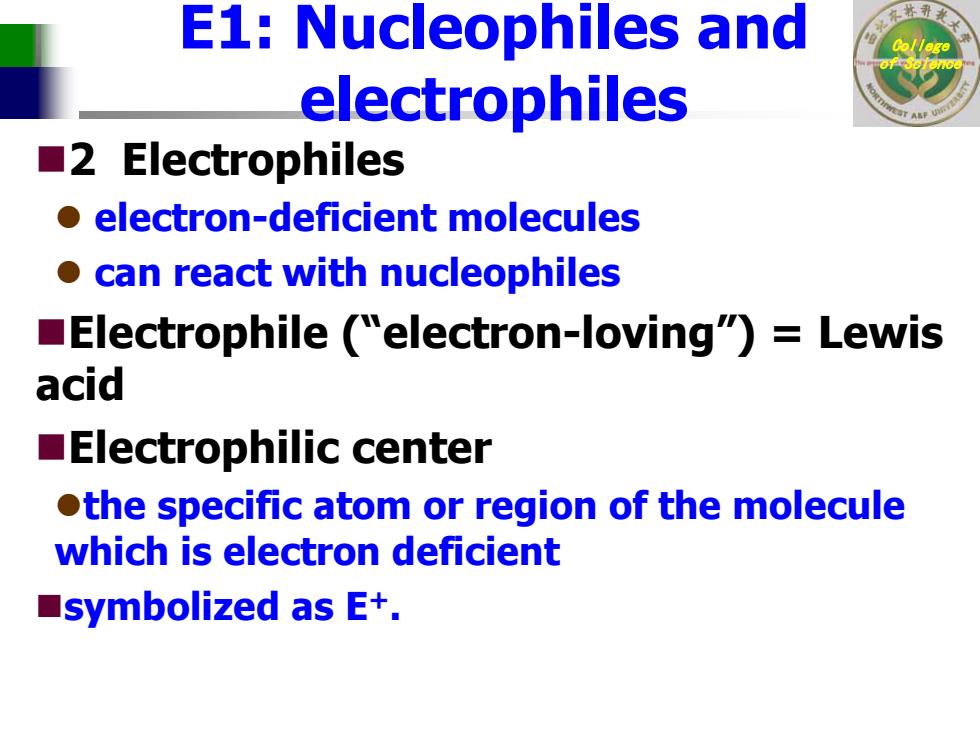
E1:Nucleophiles and electrophiles ■2 Electrophiles o electron-deficient molecules o can react with nucleophiles Electrophile ("electron-loving")=Lewis acid Electrophilic center othe specific atom or region of the molecule which is electron deficient ■symbolized as E+
College of Science E1: Nucleophiles and electrophiles 2 Electrophiles z electron-deficient molecules z can react with nucleophiles Electrophile (“electron-loving”) = Lewis acid Electrophilic center zthe specific atom or region of the molecule which is electron deficient symbolized as E +