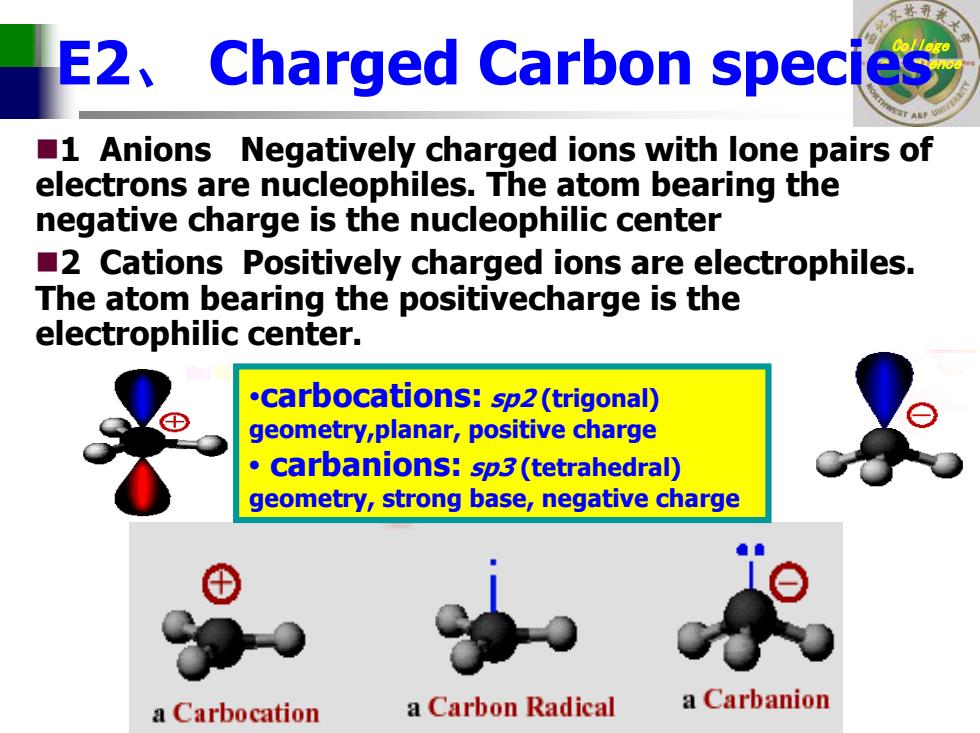
E2 Charged Carbon species 1 Anions Negatively charged ions with lone pairs of electrons are nucleophiles.The atom bearing the negative charge is the nucleophilic center 2 Cations Positively charged ions are electrophiles. The atom bearing the positivecharge is the electrophilic center. .carbocations:sp2(trigonal) geometry,planar,positive charge carbanions:sp3(tetrahedral) geometry,strong base,negative charge a Carbocation a Carbon Radical a Carbanion
College E2、 Charged Carbon speciesof Science 1 Anions Negatively charged ions with lone pairs of electrons are nucleophiles. The atom bearing the negative charge is the nucleophilic center 2 Cations Positively charged ions are electrophiles. The atom bearing the positivecharge is the electrophilic center. •carbocations: sp2 (trigonal) geometry,planar, positive charge • carbanions: sp3 (tetrahedral) geometry, strong base, negative charge
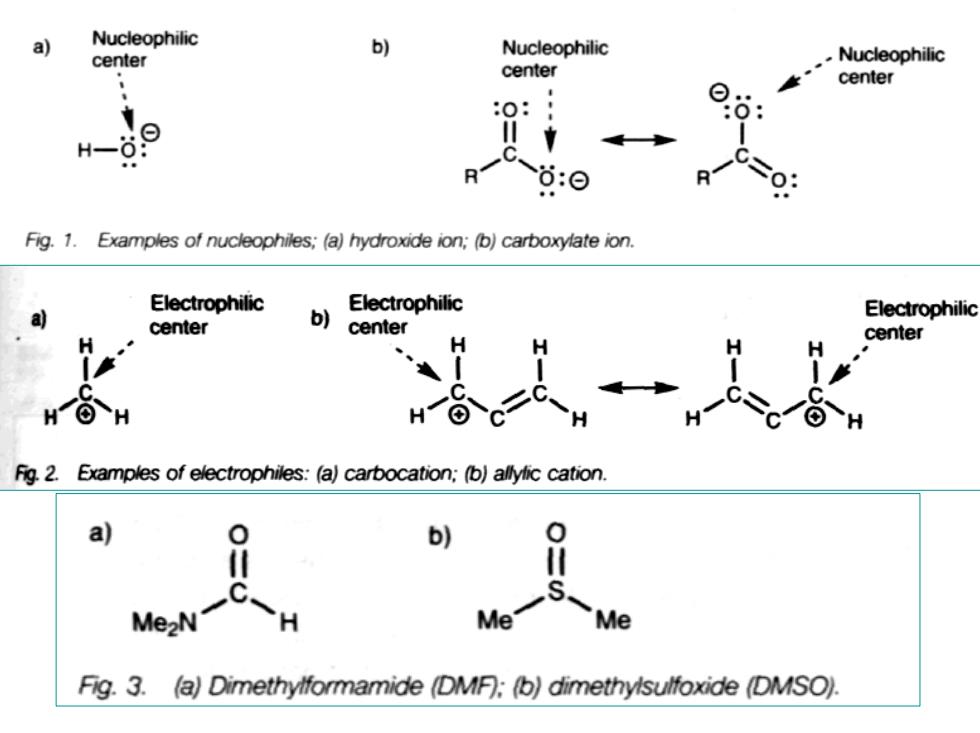
a Nucleophilic , Nucleophilic center .Nucleophilic center center 9 :⊙ Fig.1.Examples of nucleophiles;(a)hydroxide ion;(b)carboxylate ion. Electrophilic Electrophilic a) center b) Electrophilic center H Fig.2. Examples of electrophiles:(a)carbocation;(b)allylic cation. a 0 b) Me2N C-H Me- Me Fig.3.(a)Dimethylformamide (DMF):(b)dimethylsulfoxide (DMSO)
College of Science

E3-1Nu:(亲核试剂与亲核性 Relative Nucleophilicity ■ 亲核性:在过渡态时对缺电子碳亲合能力(亲 近C+) ■碱性:与质子结合能力(亲近H+) 亲核能力: ■ RS>CN->I>NH3(NH2R )>RO (OH) >Br>PhO>CI》H20>F
College of Science E3-1 Nu: (亲核试剂与亲核性) Relative Nucleophilicity 亲核性:在过渡态时对缺电子碳亲合能力(亲 近C+) 碱性: 与质子结合能力(亲近H+) 亲核能力: RS->CN->I->NH3(NH2R )>RO- (OH-) >Br- >PhO- >Cl- 》H2O >F-