
Substitution and Elimination Reactions of Alkyl Halides
Substitution and Elimination Reactions of Alkyl Halides
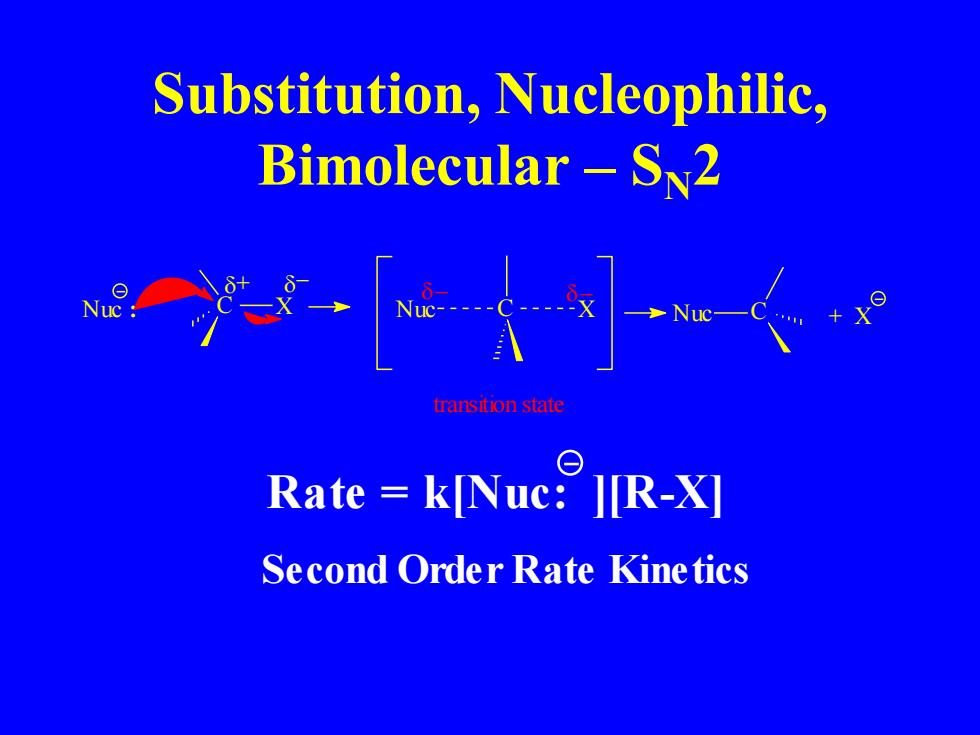
Substitution,Nucleophilic, Bimolecular-S2 一人]人 transition state Rate k[Nuc:][R-X] Second Order Rate Kinetics
Substitution, Nucleophilic, Bimolecular – SN2 C X + − Nuc : Nuc C X Nuc C + X transition state − − Rate = k[Nuc: ][R-X] Second Order Rate Kinetics
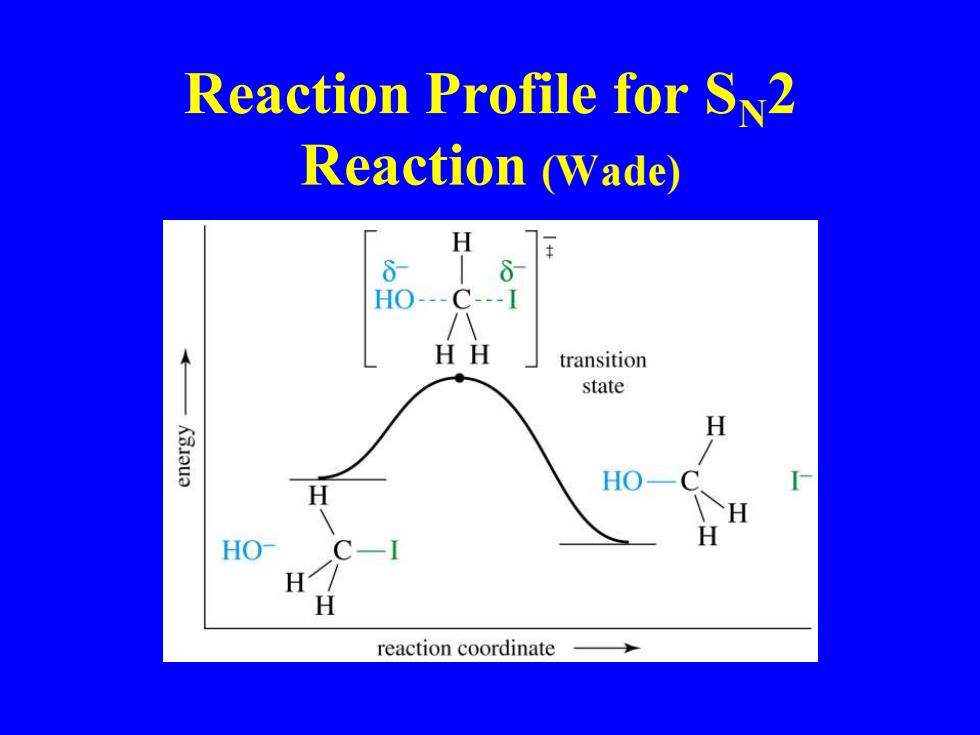
Reaction Profile for Sv2 Reaction (Wade) H 8 HO---C---I HH transition state H K.oua HO H reaction coordinate
Reaction Profile for SN2 Reaction (Wade)
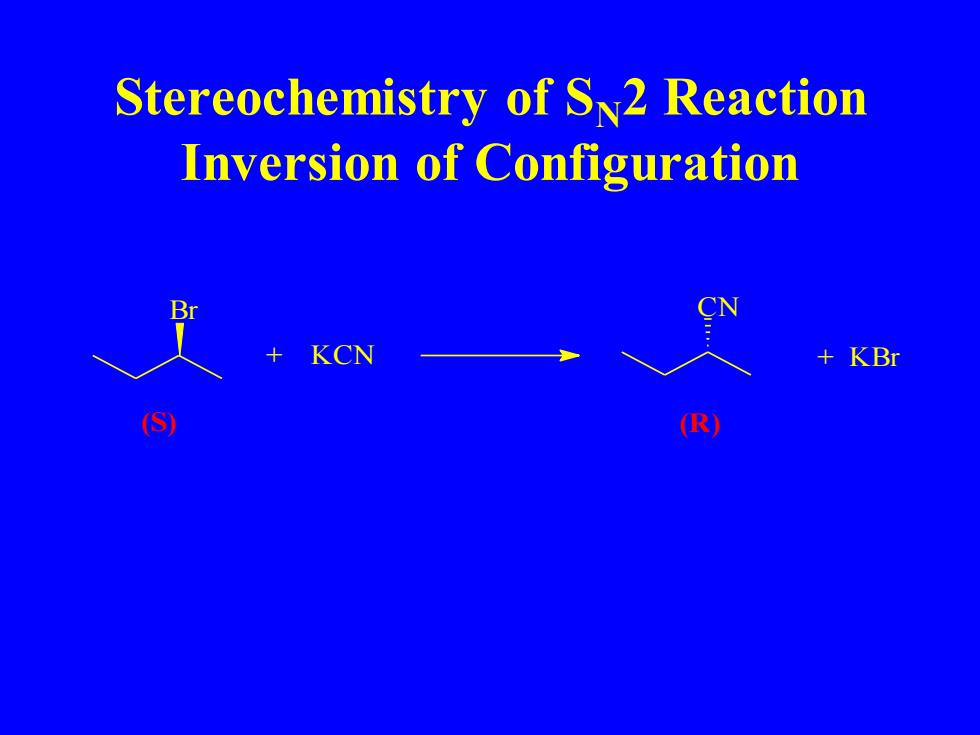
Stereochemistry of S2 Reaction Inversion of Configuration KCN KBr S R
Stereochemistry of SN2 Reaction Inversion of Configuration Br + KCN CN + KBr (S) (R)
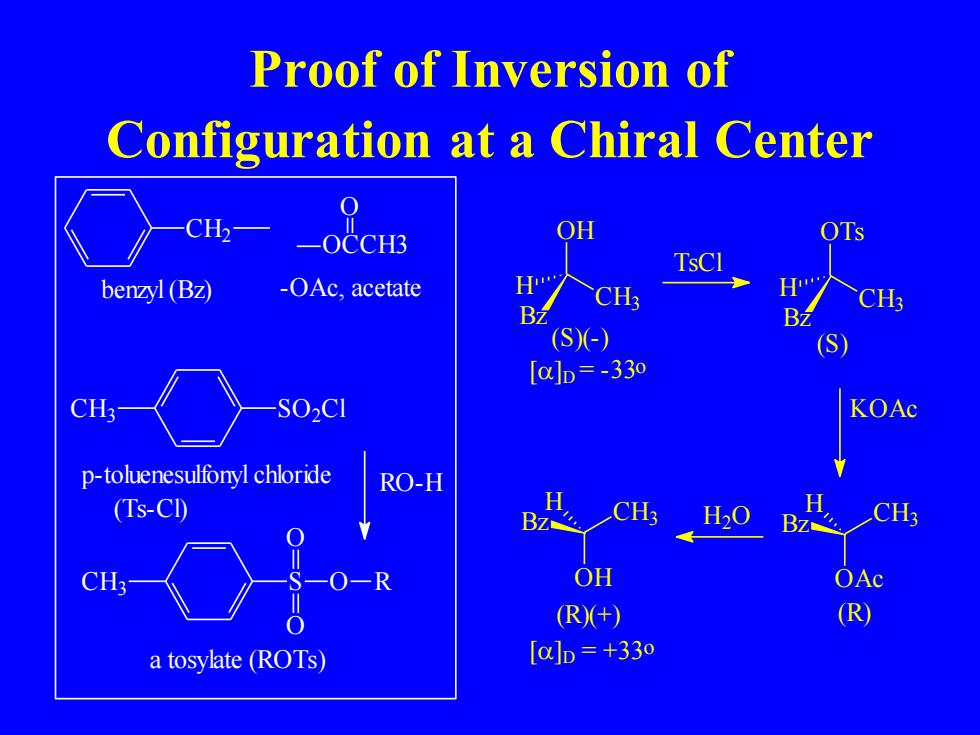
Proof of Inversion of Configuration at a Chiral Center CH2一 _OCCH3 TsCl benzyl (BZ) -OAc,acetate 6m.. CH CH; BZ (S(-) (S [a]D=-33o CH3- S02C1 KOAc p-toluenesulfonyl chloride RO-H (Ts-CI) BZ CH H20 CH3- -R OH OAc (R(+) R) a tosylate (ROTs) [a]D=+33o
Proof of Inversion of Configuration at a Chiral Center CH2 benzyl (Bz) CH SO2Cl 3 p-toluenesulfonyl chloride (Ts-Cl) CH3 S O O O R RO-H a tosylate (ROTs) OH CH3 Bz H []D = -33o (S)(-) TsCl OTs CH3 Bz H (S) KOAc OCCH3 O -OAc, acetate OAc Bz CH3 H (R) OH Bz CH3 H []D = +33o (R)(+) H2O