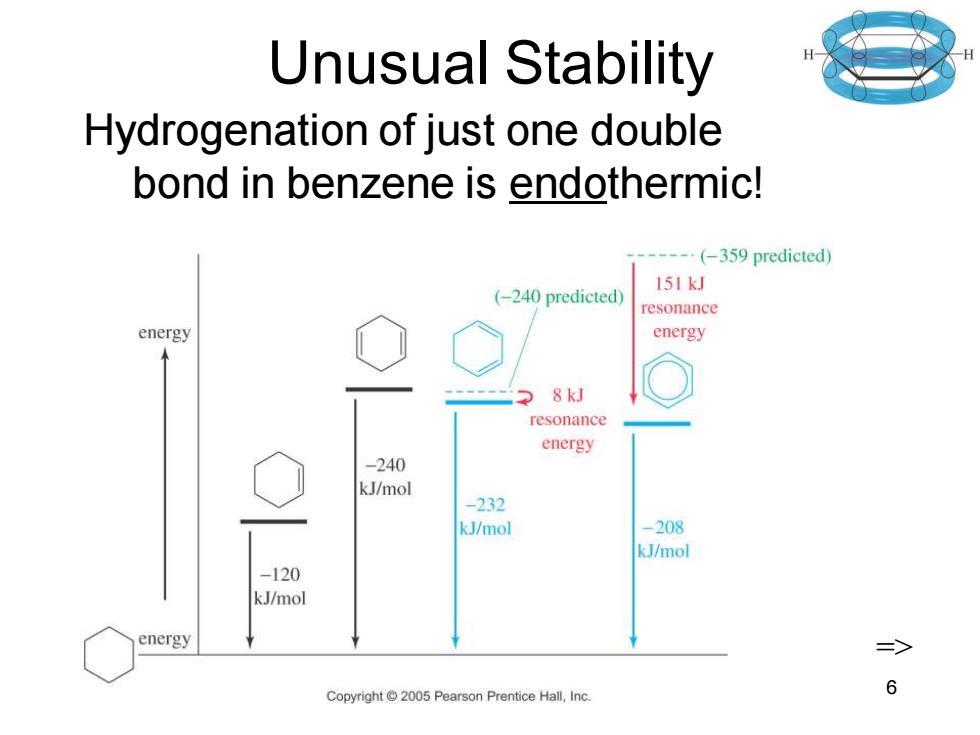
Unusual Stability Hydrogenation of just one double bond in benzene is endothermic! --…(-359 predicted) 151kJ (-240 predicted) resonance energy energy 8kJ resonance energy -240 kJ/mol -232 kJ/mol -208 kJ/mol -120 kJ/mol energy Copyright 2005 Pearson Prentice Hall,Inc. 6
Chapter 16 6 Unusual Stability Hydrogenation of just one double bond in benzene is endothermic! =>
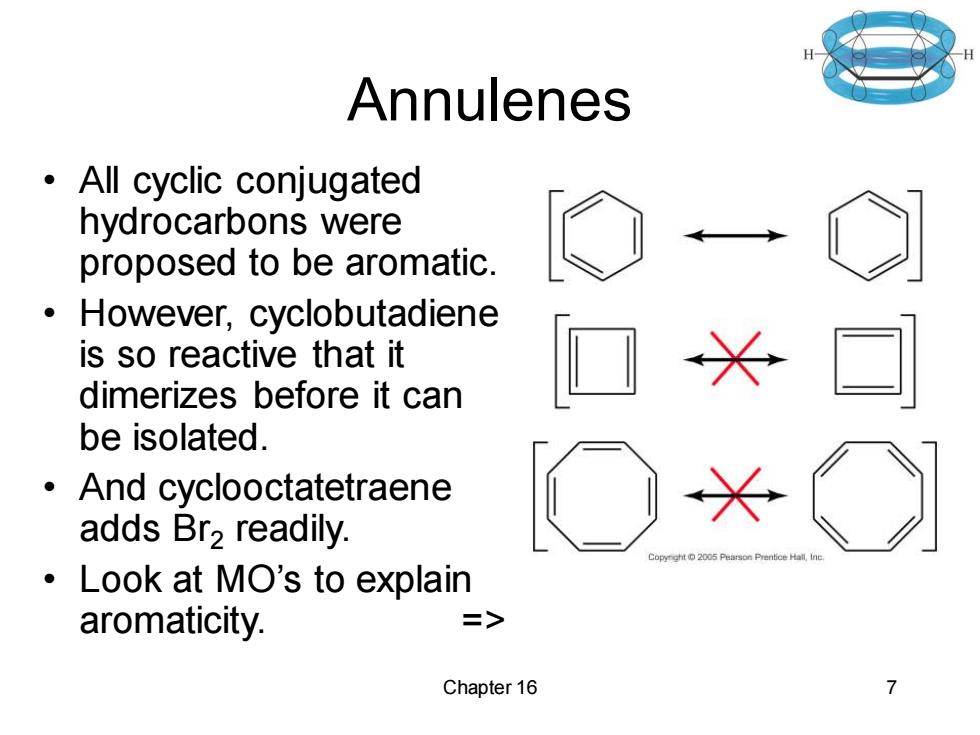
Annulenes All cyclic conjugated hydrocarbons were proposed to be aromatic. However,cyclobutadiene is so reactive that it dimerizes before it can be isolated. And cyclooctatetraene adds Br2 readily. Pea:son Prentice Hall, Look at MO's to explain aromaticity. => Chapter 16 7
Chapter 16 7 Annulenes • All cyclic conjugated hydrocarbons were proposed to be aromatic. • However, cyclobutadiene is so reactive that it dimerizes before it can be isolated. • And cyclooctatetraene adds Br2 readily. • Look at MO’s to explain aromaticity. =>

MO Rules for Benzene Six overlapping p orbitals must form six molecular orbitals. Three will be bonding,three antibonding. Lowest energy MO will have all bonding interactions,no nodes. As energy of MO increases,the number of nodes increases. 二> Chapter 16 8
Chapter 16 8 MO Rules for Benzene • Six overlapping p orbitals must form six molecular orbitals. • Three will be bonding, three antibonding. • Lowest energy MO will have all bonding interactions, no nodes. • As energy of MO increases, the number of nodes increases. =>
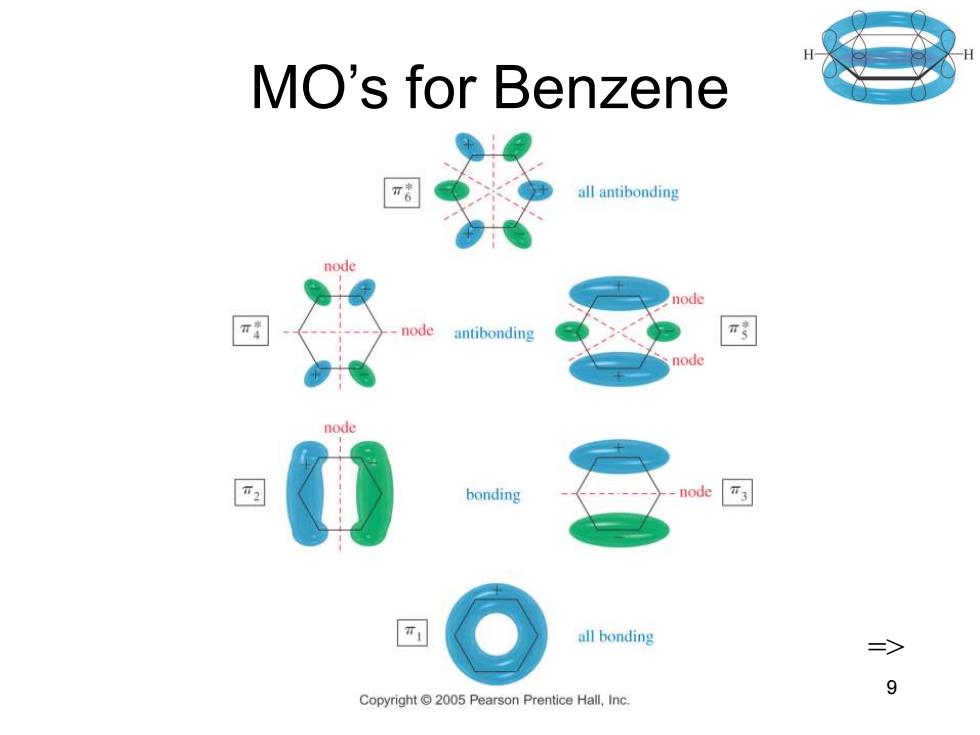
MO's for Benzene all antibonding node .node antibonding node bonding ode all bonding => 9 Copyright2005 Pearson Prentice Hall,Inc
Chapter 16 9 MO’s for Benzene =>
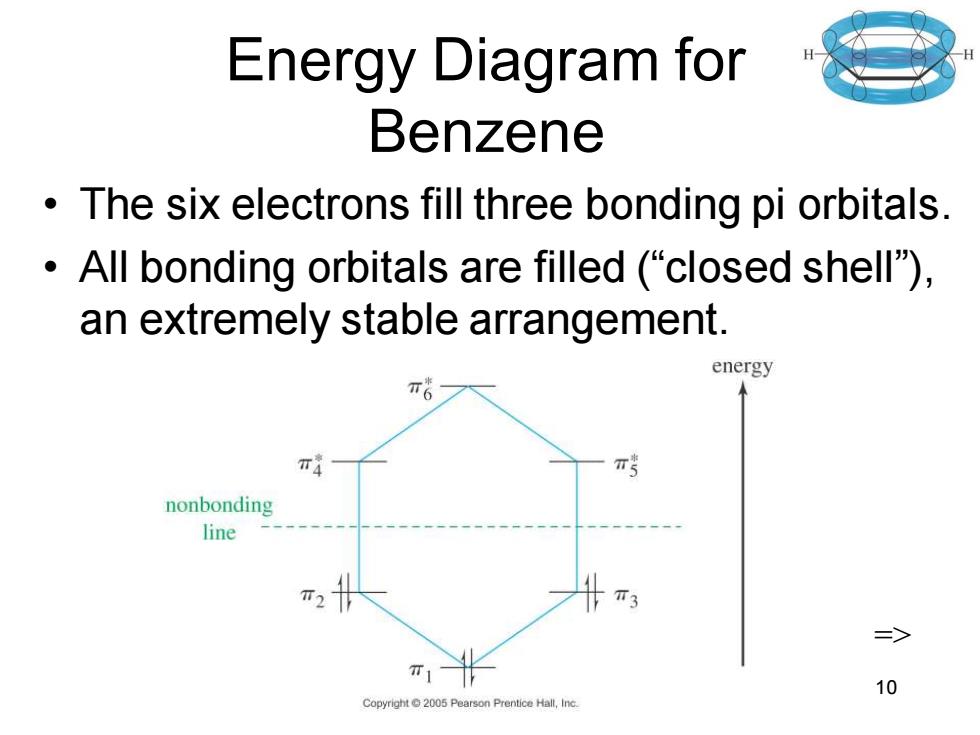
Energy Diagram for Benzene The six electrons fill three bonding pi orbitals. All bonding orbitals are filled ("closed shell). an extremely stable arrangement. energy π4 nonbonding line T3 三> 10 Copyright2005 Pearson Prentice Hall,Inc
Chapter 16 10 Energy Diagram for Benzene • The six electrons fill three bonding pi orbitals. • All bonding orbitals are filled (“closed shell”), an extremely stable arrangement. =>