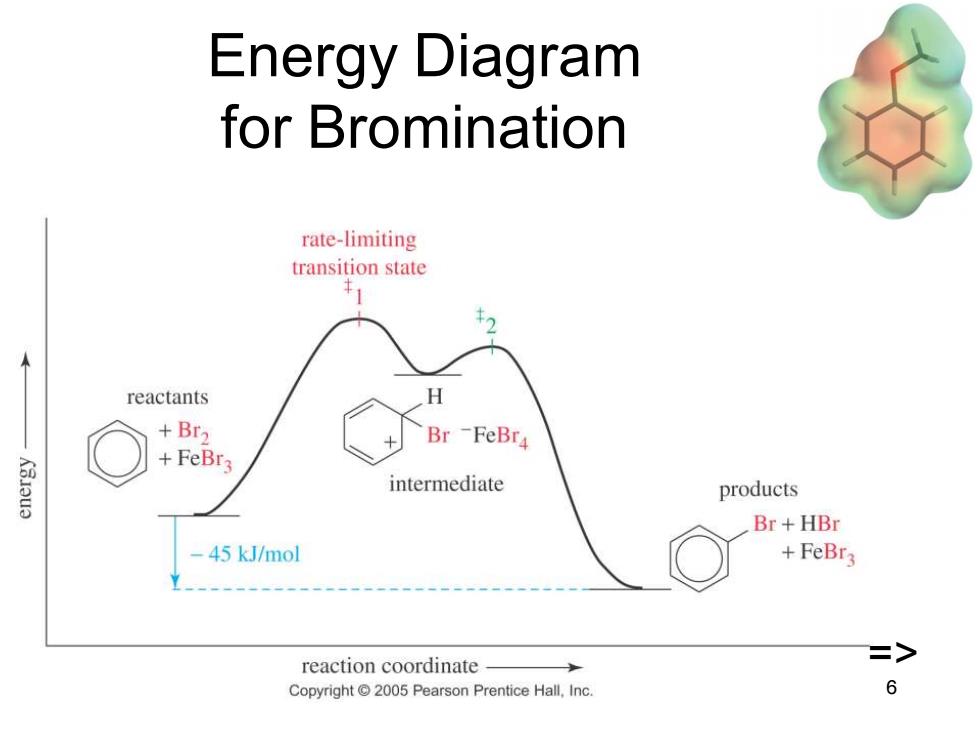
Energy Diagram for Bromination rate-limiting transition state reactants H +Br2 Br-FeBr4 +FeBr3 Kau3 intermediate products Br+HBr 45 kJ/mol +FeBr3 > reaction coordinate Copyright 2005 Pearson Prentice Hall,Inc 6
Chapter 17 6 Energy Diagram for Bromination =>
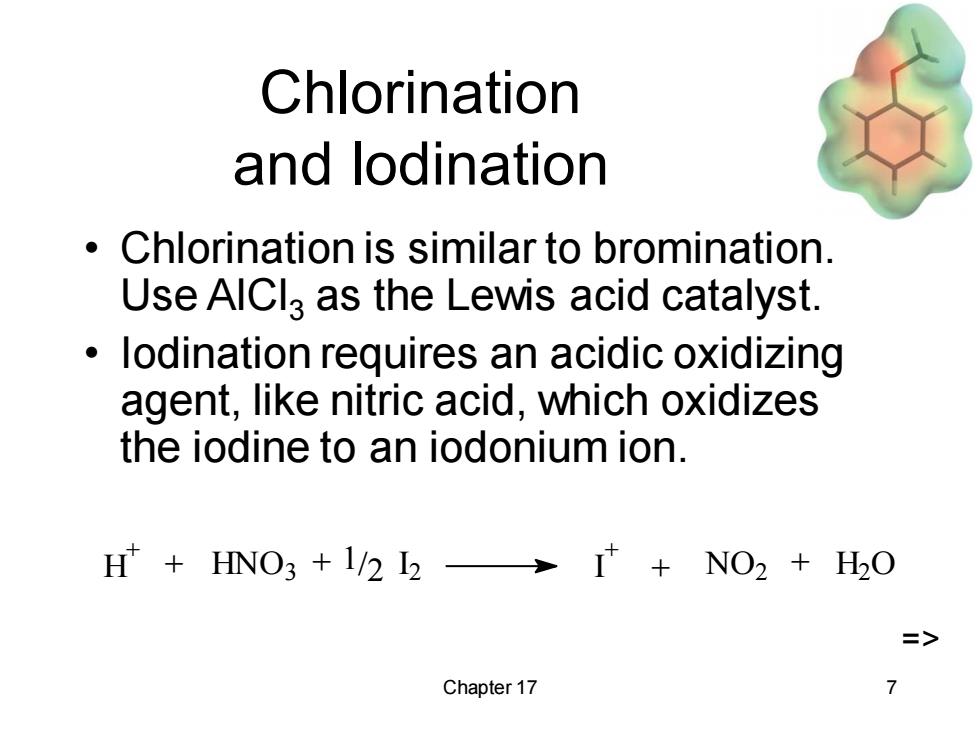
Chlorination and lodination 。 Chlorination is similar to bromination. Use AIClg as the Lewis acid catalyst. lodination requires an acidic oxidizing agent,like nitric acid,which oxidizes the iodine to an iodonium ion. H HNO3 +1/2 I2>I NO2 H2O => Chapter 17 7
Chapter 17 7 Chlorination and Iodination • Chlorination is similar to bromination. Use AlCl3 as the Lewis acid catalyst. • Iodination requires an acidic oxidizing agent, like nitric acid, which oxidizes the iodine to an iodonium ion. H + HNO3 I 2 1/2 I + + + + NO2 + H2 O =>
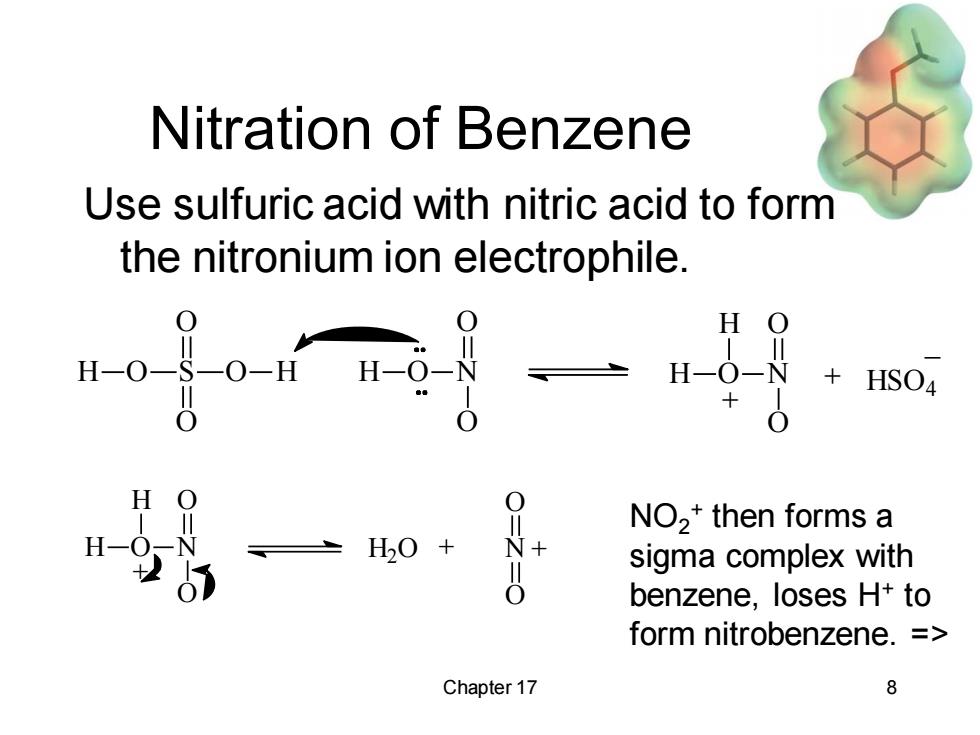
Nitration of Benzene Use sulfuric acid with nitric acid to form the nitronium ion electrophile. H H HSO4 0 ,0+ N NO2*then forms a sigma complex with benzene,loses H+to form nitrobenzene.= Chapter 17 8
Chapter 17 8 Nitration of Benzene Use sulfuric acid with nitric acid to form the nitronium ion electrophile. H O N O O H O S O H O O + HSO4 _ H O N H O O + H O N H O O + H2O + N O O + NO2 + then forms a sigma complex with benzene, loses H+ to form nitrobenzene. =>

Sulfonation Sulfur trioxide,SO3,in fuming sulfuric acid is the electrophile. benzenesulfonic acid Chapter 17 9
Chapter 17 9 Sulfonation Sulfur trioxide, SO3 , in fuming sulfuric acid is the electrophile. S O O O S O O O S O O O S O O O + + + _ _ _ S O O O H S O O O H + _ S HO O O benzenesulfonic acid =>
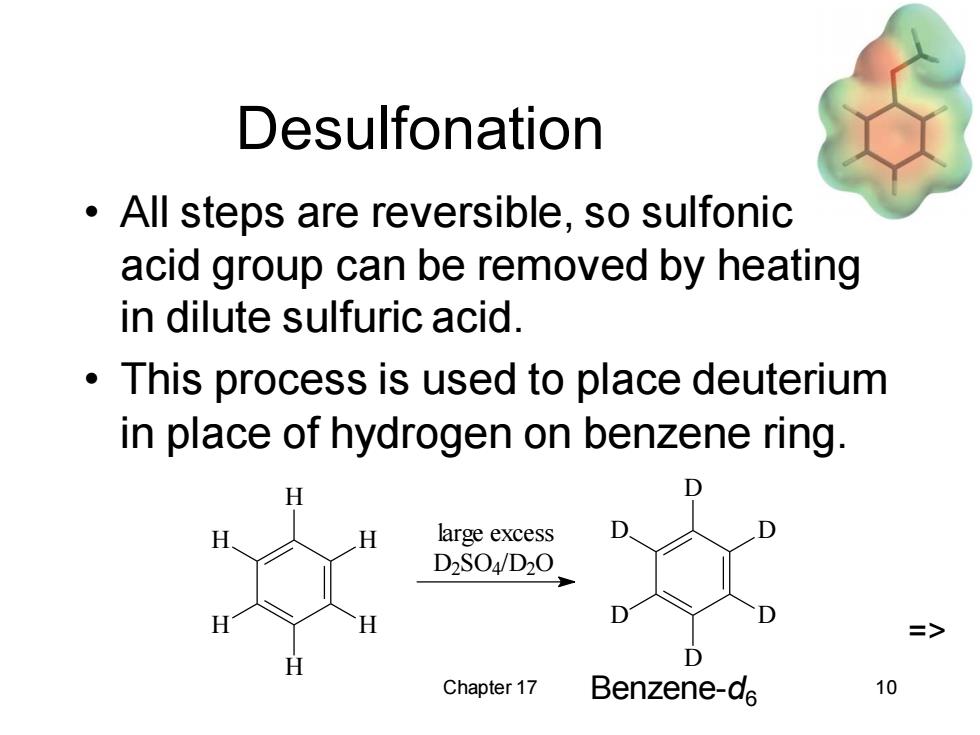
Desulfonation All steps are reversible,so sulfonic acid group can be removed by heating in dilute sulfuric acid. This process is used to place deuterium in place of hydrogen on benzene ring. H large excess D2S04/D20. => H Chapter 17 Benzene-d6 10
Chapter 17 10 Desulfonation • All steps are reversible, so sulfonic acid group can be removed by heating in dilute sulfuric acid. • This process is used to place deuterium in place of hydrogen on benzene ring. Benzene-d6 => D D D D D D D2SO4/D2O large excess H H H H H H