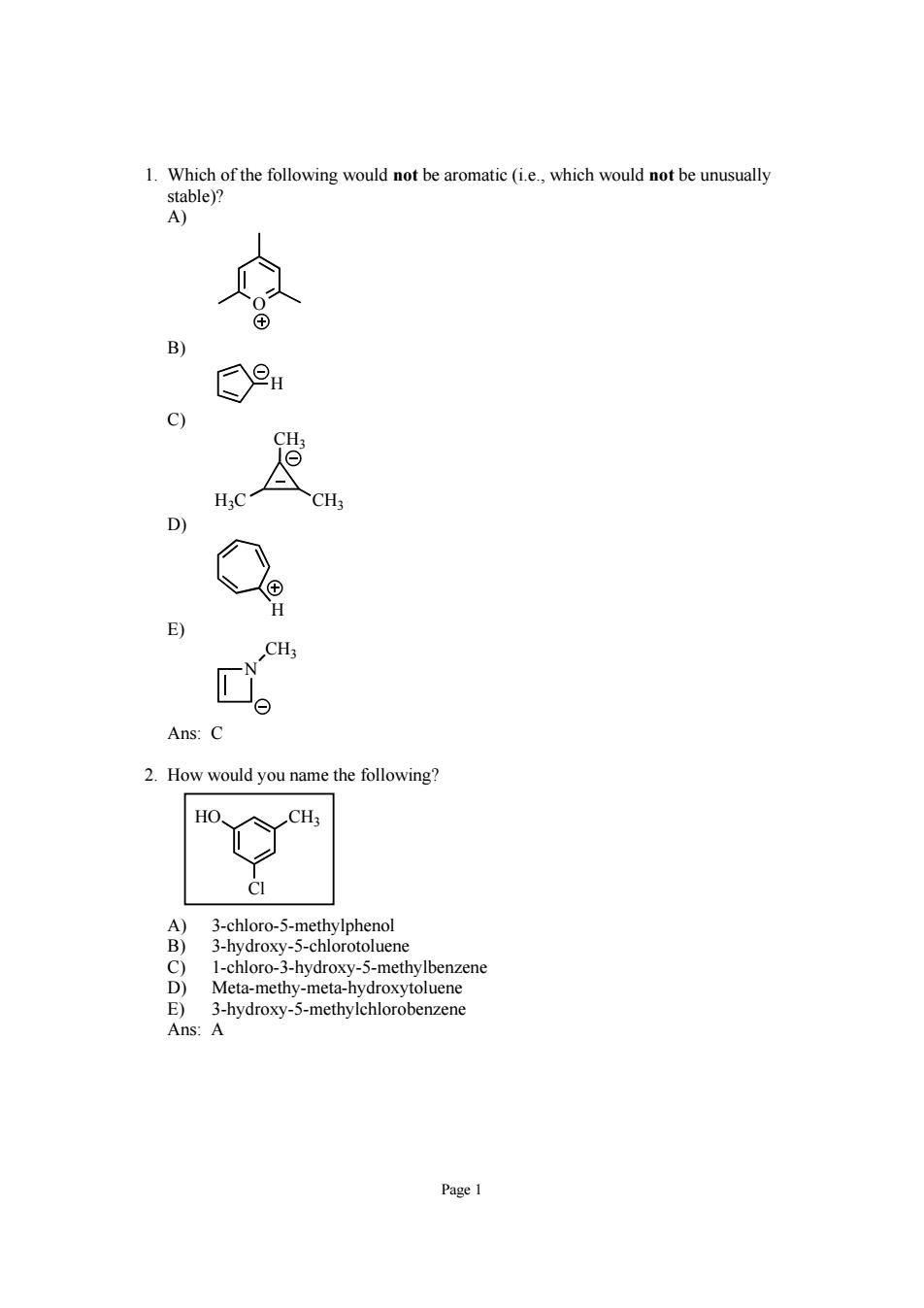
1.Which of the following would not be aromatic (i.e..which would not be unusually 0 CH Ans:C 2.How would you name the following? HO CH; 8 1-chloro-3-hydroxy-5-methylbenzene Meta-methy-meta-hydroxytoluene 3-hydroxy-5-methylchlorobenzene Ans:A Page 1
Page 1 1. Which of the following would not be aromatic (i.e., which would not be unusually stable)? A) O B) H C) H3C CH3 CH3 D) H E) N CH3 Ans: C 2. How would you name the following? HO CH3 Cl A) 3-chloro-5-methylphenol B) 3-hydroxy-5-chlorotoluene C) 1-chloro-3-hydroxy-5-methylbenzene D) Meta-methy-meta-hydroxytoluene E) 3-hydroxy-5-methylchlorobenzene Ans: A
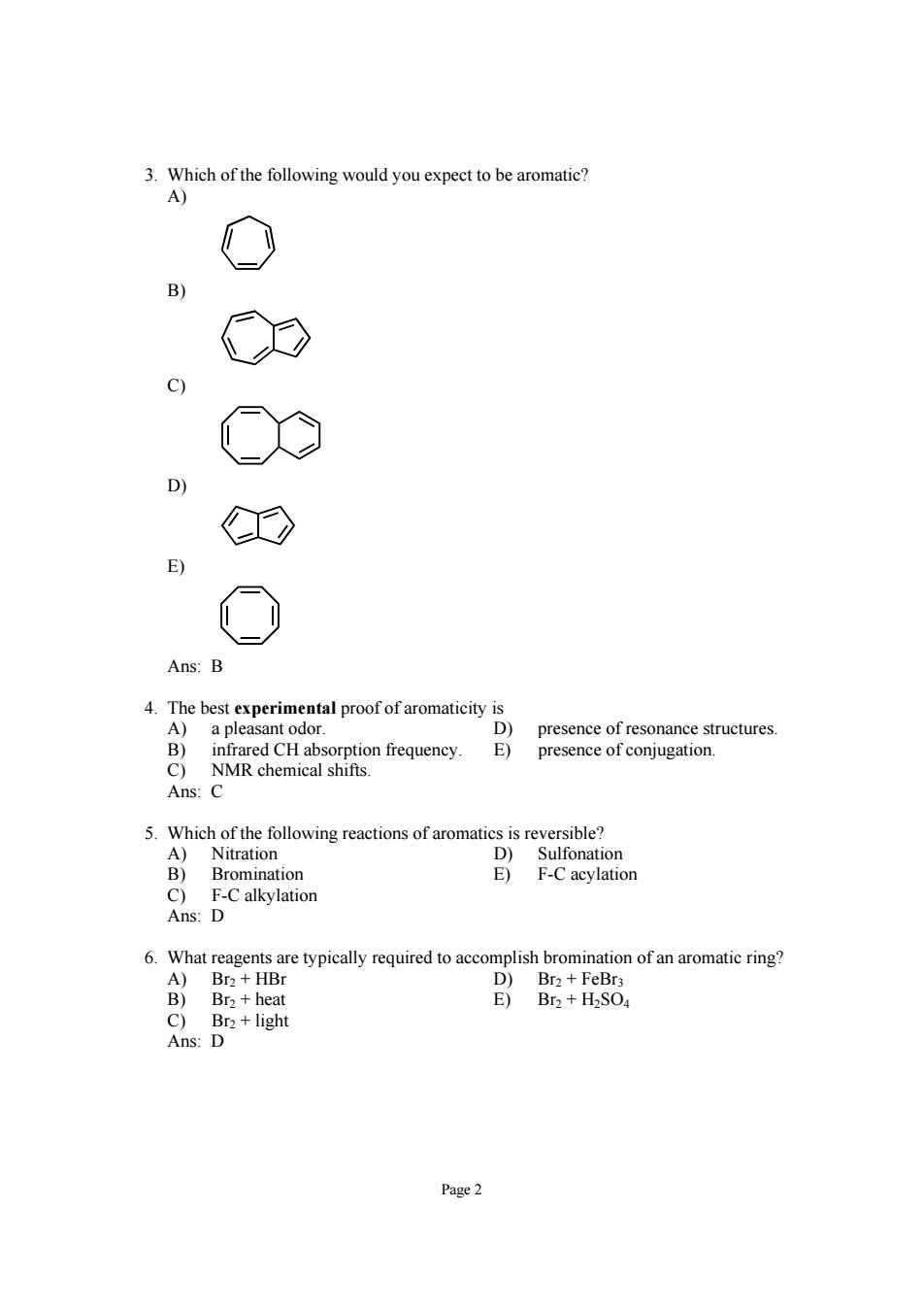
3.Which of the following would you expect to be aromatic? A) C E Ans:B 兰fof红mi presence of resonance structures presence of conjugation Ans: 5.Which of the following reactions of aromatics is reversible? A)Nitration D)Sulfonation B)Bromination F-C acylation 6.What reagents are typically required to accomplish bromination of an aromatic ring? A)Br2+HBr D)Br2+FeBrs B)Br2+heat E)Br2+H2SOa C)Br2+light Ans:D Page2
Page 2 3. Which of the following would you expect to be aromatic? A) B) C) D) E) Ans: B 4. The best experimental proof of aromaticity is A) a pleasant odor. D) presence of resonance structures. B) infrared CH absorption frequency. E) presence of conjugation. C) NMR chemical shifts. Ans: C 5. Which of the following reactions of aromatics is reversible? A) Nitration D) Sulfonation B) Bromination E) F-C acylation C) F-C alkylation Ans: D 6. What reagents are typically required to accomplish bromination of an aromatic ring? A) Br2 + HBr D) Br2 + FeBr3 B) Br2 + heat E) Br2 + H2SO4 C) Br2 + light Ans: D
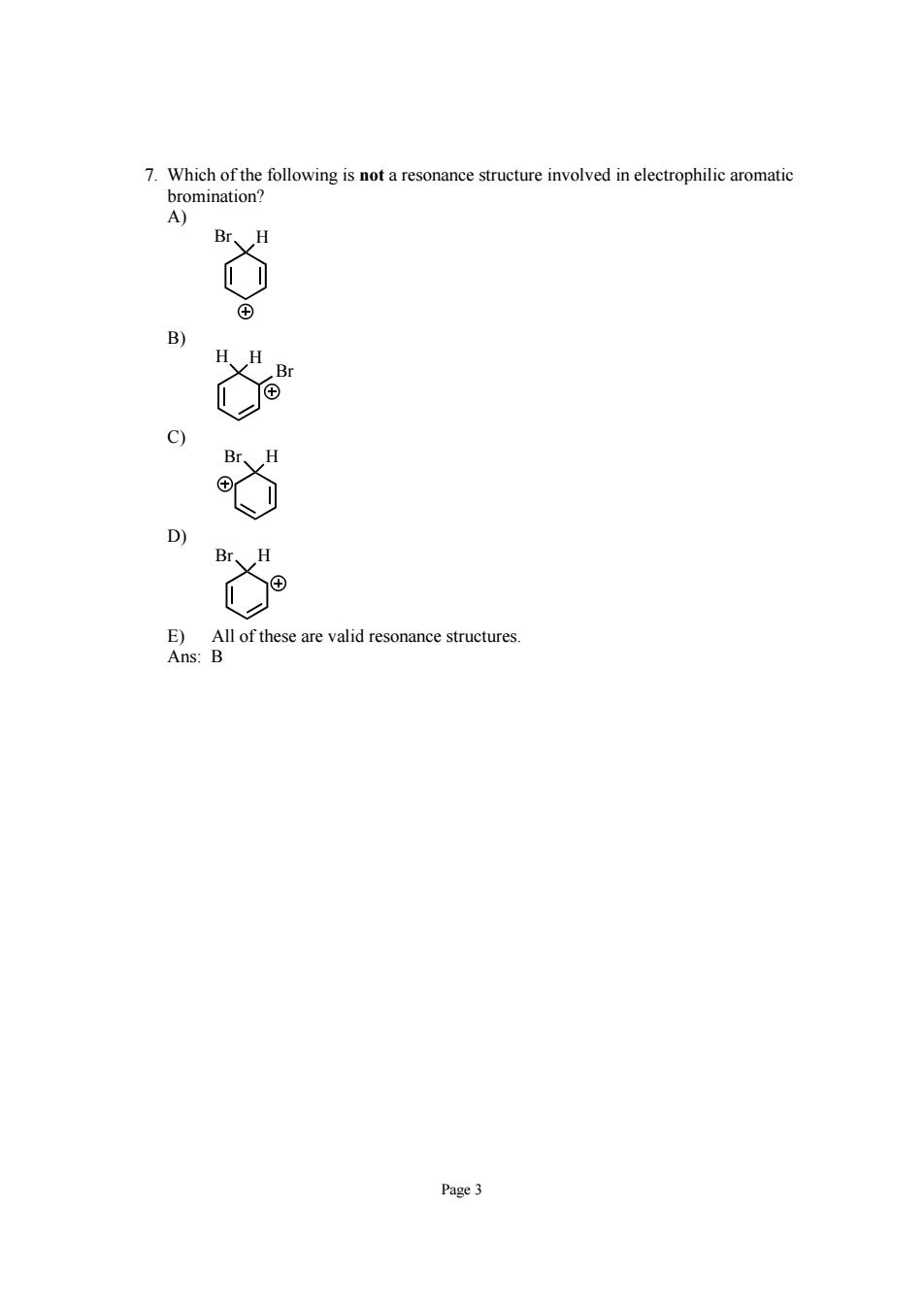
7.Which of the following is not a resonance structure involved in electrophilic aromatic B) c) ll of these are valid resonance Page3
Page 3 7. Which of the following is not a resonance structure involved in electrophilic aromatic bromination? A) Br H B) Br H H C) Br H D) Br H E) All of these are valid resonance structures. Ans: B

8.What would be the product of the following reaction? 1.BH吟HF CH3 2.H2O2,NaOH OH ne)-cn s◇a HO E)No reaction occurs Ans:E Page4
Page 4 8. What would be the product of the following reaction? CH3 H3C ? 1. BH3昑HF 2. H2O2, NaOH A) CH3 H3C OH B) CH3 H3C OH C) OH CH3 H3C D) CH3 H3C HO E) No reaction occurs Ans: E
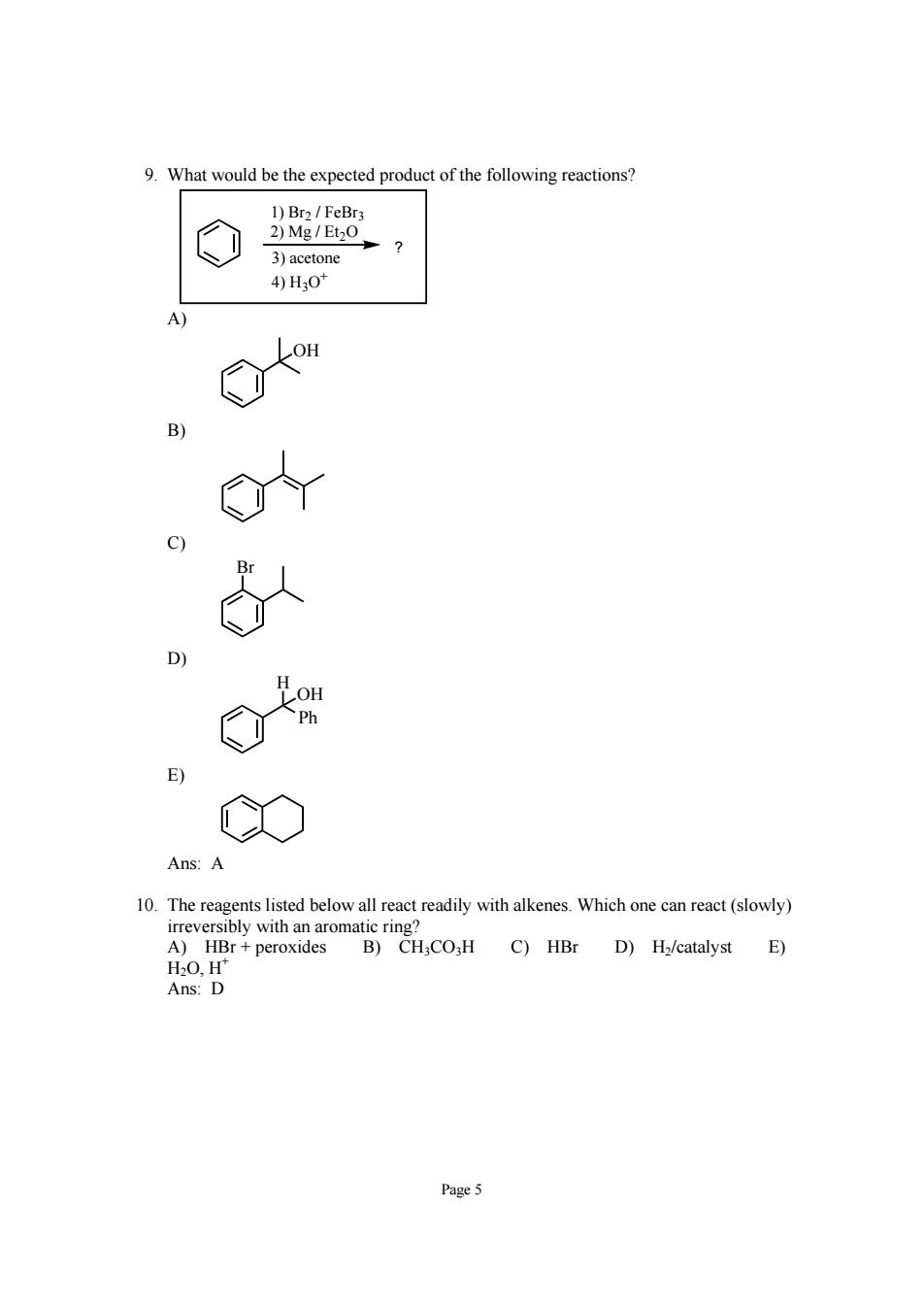
9.What would be the expected product of the following reactions? 1)Br2/FeBrs 2)Mg/Et2O 3)acetone 4)H30* 3 Ans:A A)Hrperoxides B)CH,CO,H C)HBr D)H/catalyst E) H20,H Ans:D Page5
Page 5 9. What would be the expected product of the following reactions? ? 3) acetone 4) H3O+ 2) Mg / Et2O 1) Br2 / FeBr3 A) OH B) C) Br D) OH Ph H E) Ans: A 10. The reagents listed below all react readily with alkenes. Which one can react (slowly) irreversibly with an aromatic ring? A) HBr + peroxides B) CH3CO3H C) HBr D) H2/catalyst E) H2O, H+ Ans: D