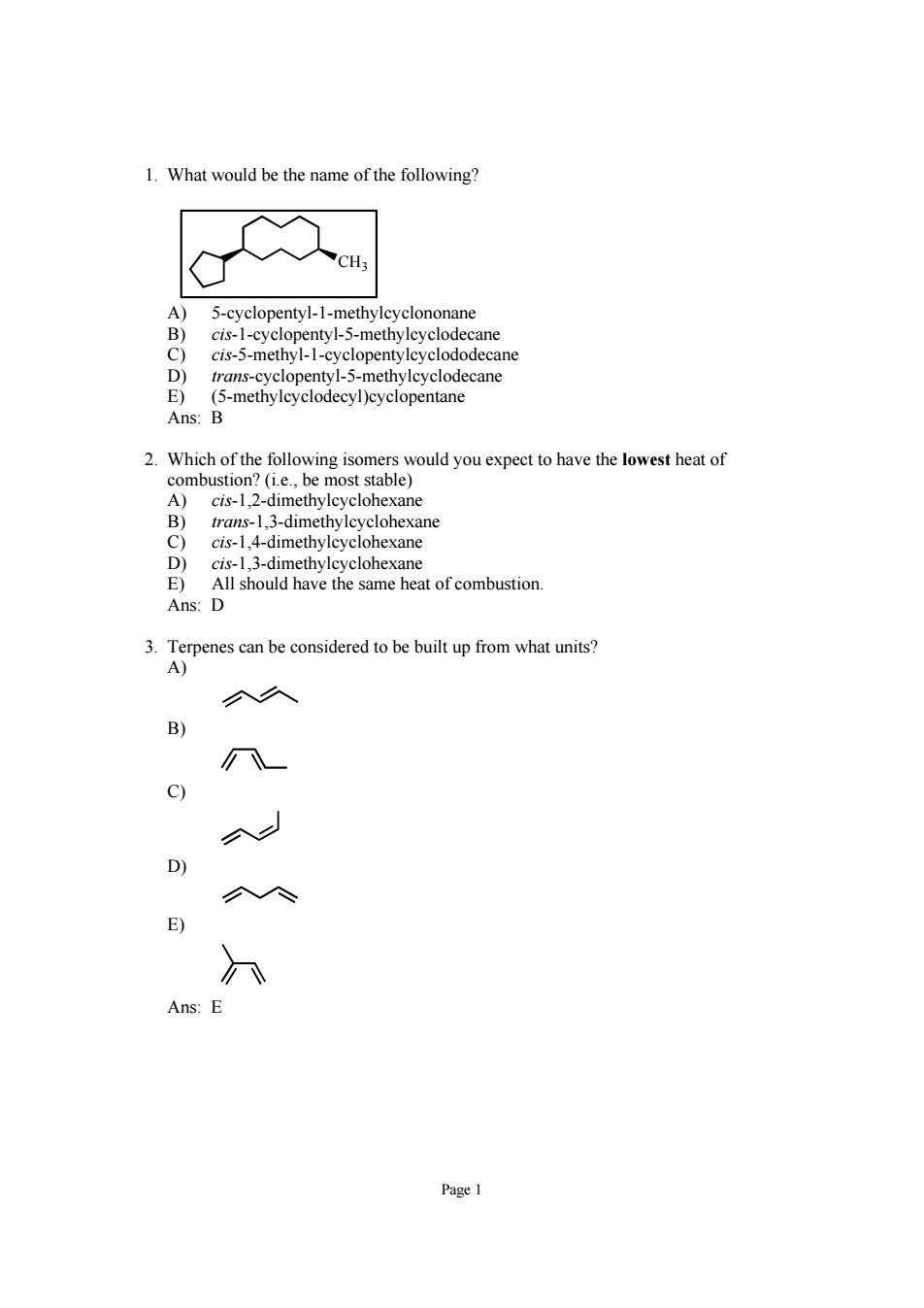
1.What would be the name of the following? 5-cyclopentyl-1-methyleyclononane cis-1-cyclopentyl-5-methylcyclodecane cis-5-methyl-1-cyclopentylcyclododecane trans-cyclopentyl-5-methylcyclodecane (5-methylcyclodecyl)cyclopentane Ans: A)cis-1,2-dimethylcyclohexane trans-1,3-dimethylcyclohexane cis-1.4-dimethyleyclohexane Ans: 3.Terpenes can be considered to be built up from what units? c) D) E) Ans:
Page 1 1. What would be the name of the following? CH3 A) 5-cyclopentyl-1-methylcyclononane B) cis-1-cyclopentyl-5-methylcyclodecane C) cis-5-methyl-1-cyclopentylcyclododecane D) trans-cyclopentyl-5-methylcyclodecane E) (5-methylcyclodecyl)cyclopentane Ans: B 2. Which of the following isomers would you expect to have the lowest heat of combustion? (i.e., be most stable) A) cis-1,2-dimethylcyclohexane B) trans-1,3-dimethylcyclohexane C) cis-1,4-dimethylcyclohexane D) cis-1,3-dimethylcyclohexane E) All should have the same heat of combustion. Ans: D 3. Terpenes can be considered to be built up from what units? A) B) C) D) E) Ans: E
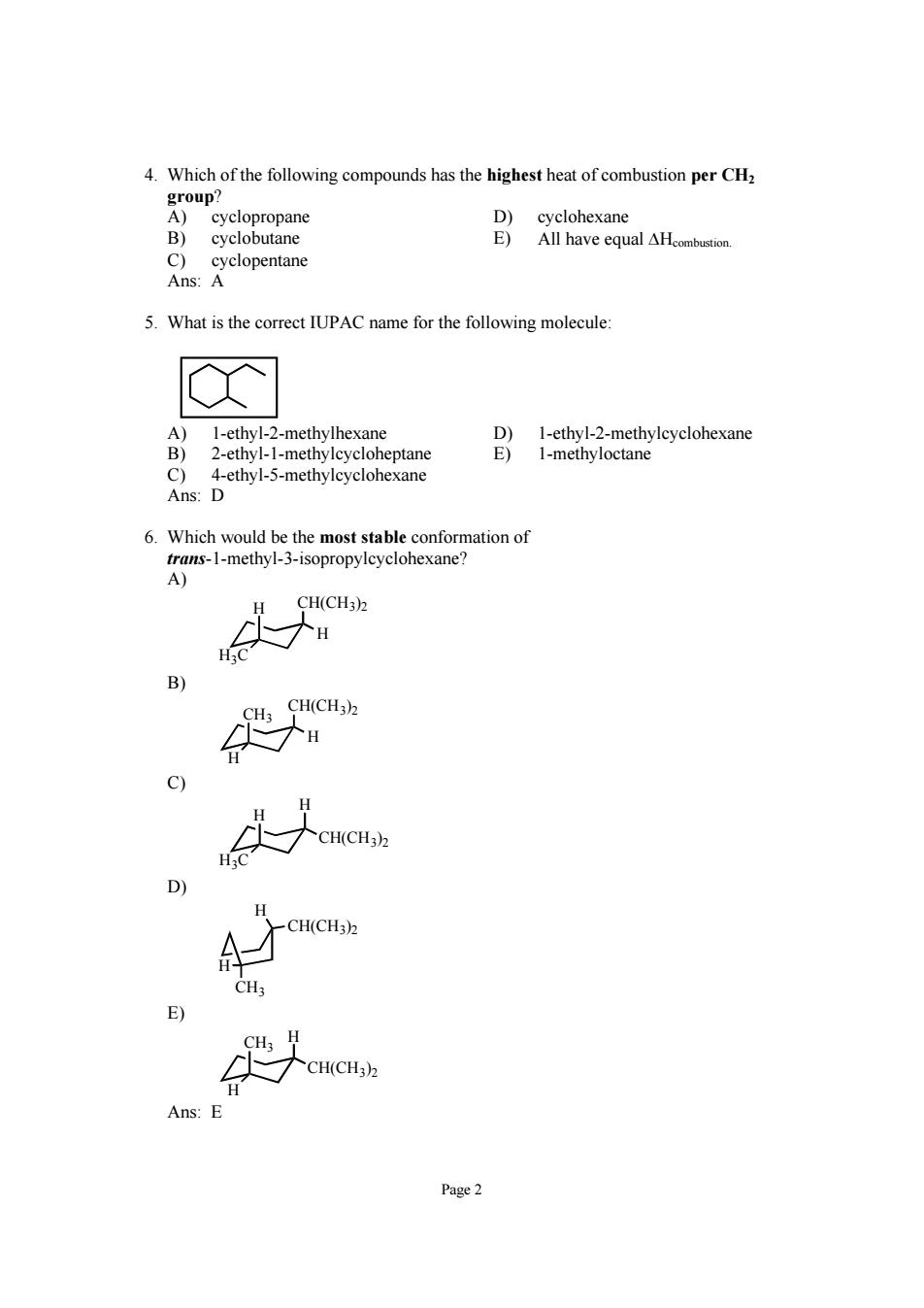
4.Which of the following compounds has the highest heat of combustion per CH cyclohexane B) 5.What is the correct IUPAC name for the following molecule: A)1-ethyl-2-methylhexane D)1-ethyl-2-methylcyclohexane 2-ethyl-1-methylcycloheptane Ans: Dethyl-5-methyleyeloe E)1-methyloctane CH(CH3) 3) 9 aa H.C D) CH(CH3). A CH(CH3) Ans:E Page2
Page 2 4. Which of the following compounds has the highest heat of combustion per CH2 group? A) cyclopropane D) cyclohexane B) cyclobutane E) All have equal ΔHcombustion. C) cyclopentane Ans: A 5. What is the correct IUPAC name for the following molecule: A) 1-ethyl-2-methylhexane D) 1-ethyl-2-methylcyclohexane B) 2-ethyl-1-methylcycloheptane E) 1-methyloctane C) 4-ethyl-5-methylcyclohexane Ans: D 6. Which would be the most stable conformation of trans-1-methyl-3-isopropylcyclohexane? A) CH(CH3) H 2 H3C H B) CH(CH3) CH 2 3 H H C) CH(CH3)2 H H3C H D) CH(CH3)2 H CH3 H E) CH H 3 H CH(CH3)2 Ans: E
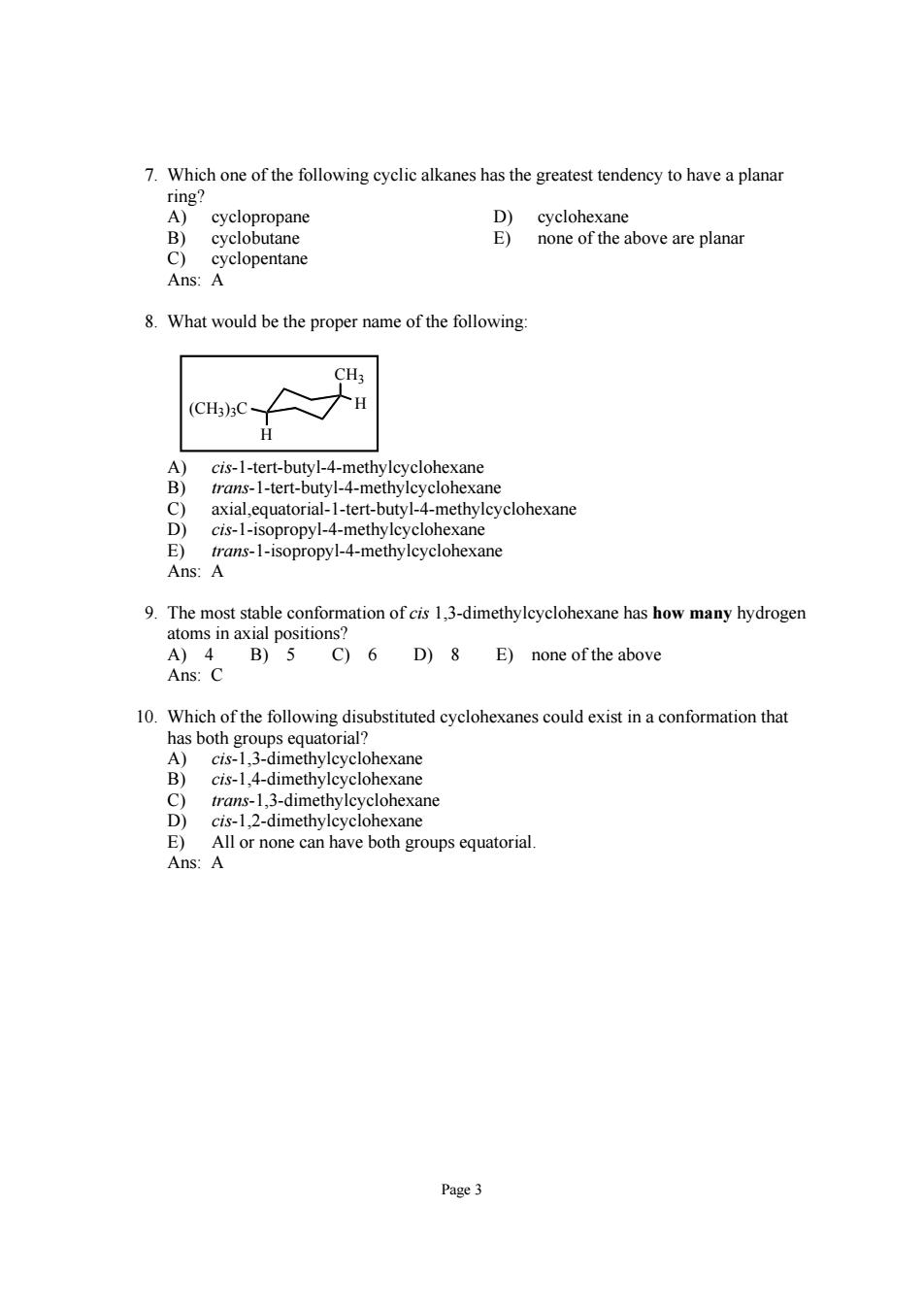
7.Which one of the following cyclic alkanes has the greatest tendency to have a planar cyclohexan none of the above are planar Ans:A 8.What would be the proper name of the following: (C)3C 才H cis-1-tert-butyl-4-methylcyclohexane trans-1-tert-butyl-4-methylcyclohexane axial.equatorial-1-tert-butyl-4-methylcyclohexane cis-1-isopropyl-4-methylcyclohexane opopy-meyleyclohe 9.The most stable conformation of cis 1.3-dimethylcyclohexane has how many hydrogen atoms in axial positions? A)4 B)5 C)6 D)8 E)none of the above Ans:C 10.Which of the following stituted cyclohexanes could exist in a conformation is-4-dimethylcyclohexane trans-1,3-dimethylcyclohexane cis-1,2-dimethylcyclohexane E) All or none can have both groups equatorial s:A Page 3
Page 3 7. Which one of the following cyclic alkanes has the greatest tendency to have a planar ring? A) cyclopropane D) cyclohexane B) cyclobutane E) none of the above are planar C) cyclopentane Ans: A 8. What would be the proper name of the following: CH3 H H (CH3)3C A) cis-1-tert-butyl-4-methylcyclohexane B) trans-1-tert-butyl-4-methylcyclohexane C) axial,equatorial-1-tert-butyl-4-methylcyclohexane D) cis-1-isopropyl-4-methylcyclohexane E) trans-1-isopropyl-4-methylcyclohexane Ans: A 9. The most stable conformation of cis 1,3-dimethylcyclohexane has how many hydrogen atoms in axial positions? A) 4 B) 5 C) 6 D) 8 E) none of the above Ans: C 10. Which of the following disubstituted cyclohexanes could exist in a conformation that has both groups equatorial? A) cis-1,3-dimethylcyclohexane B) cis-1,4-dimethylcyclohexane C) trans-1,3-dimethylcyclohexane D) cis-1,2-dimethylcyclohexane E) All or none can have both groups equatorial. Ans: A
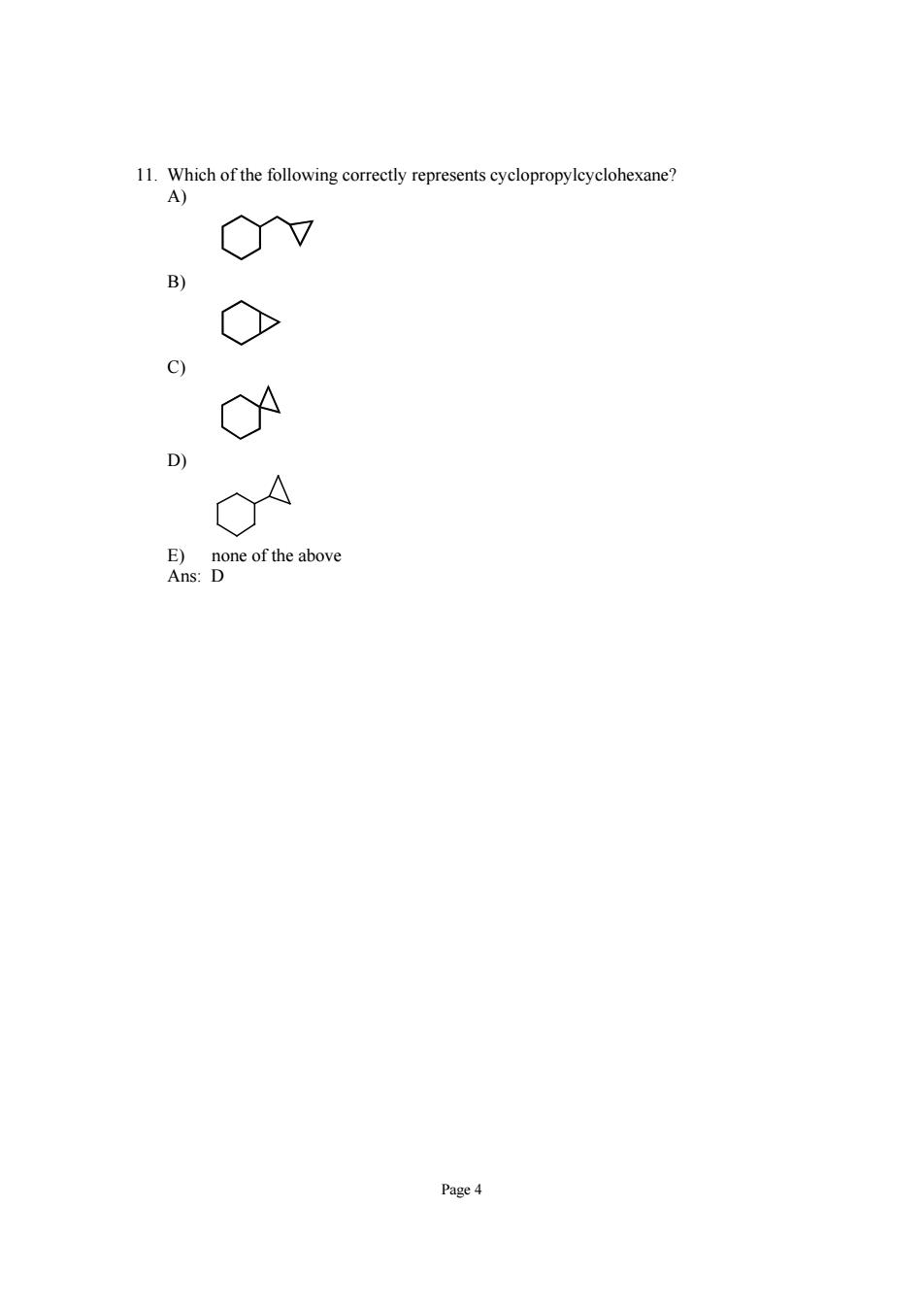
11. 0v O C) n Done of the above Page
Page 4 11. Which of the following correctly represents cyclopropylcyclohexane? A) B) C) D) E) none of the above Ans: D
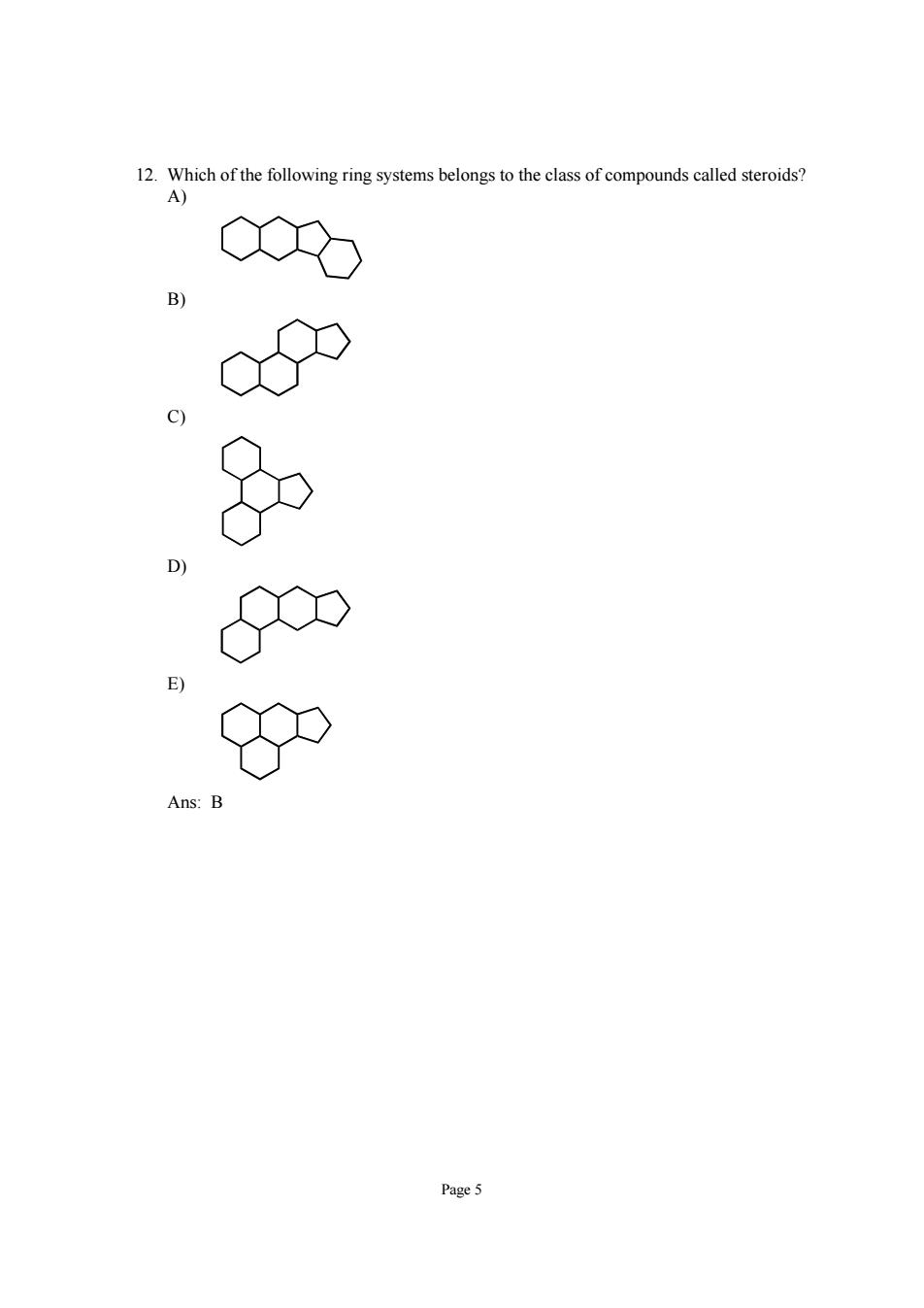
hichth olngng systems belongs to the class of compounds calld strod Page 5
Page 5 12. Which of the following ring systems belongs to the class of compounds called steroids? A) B) C) D) E) Ans: B