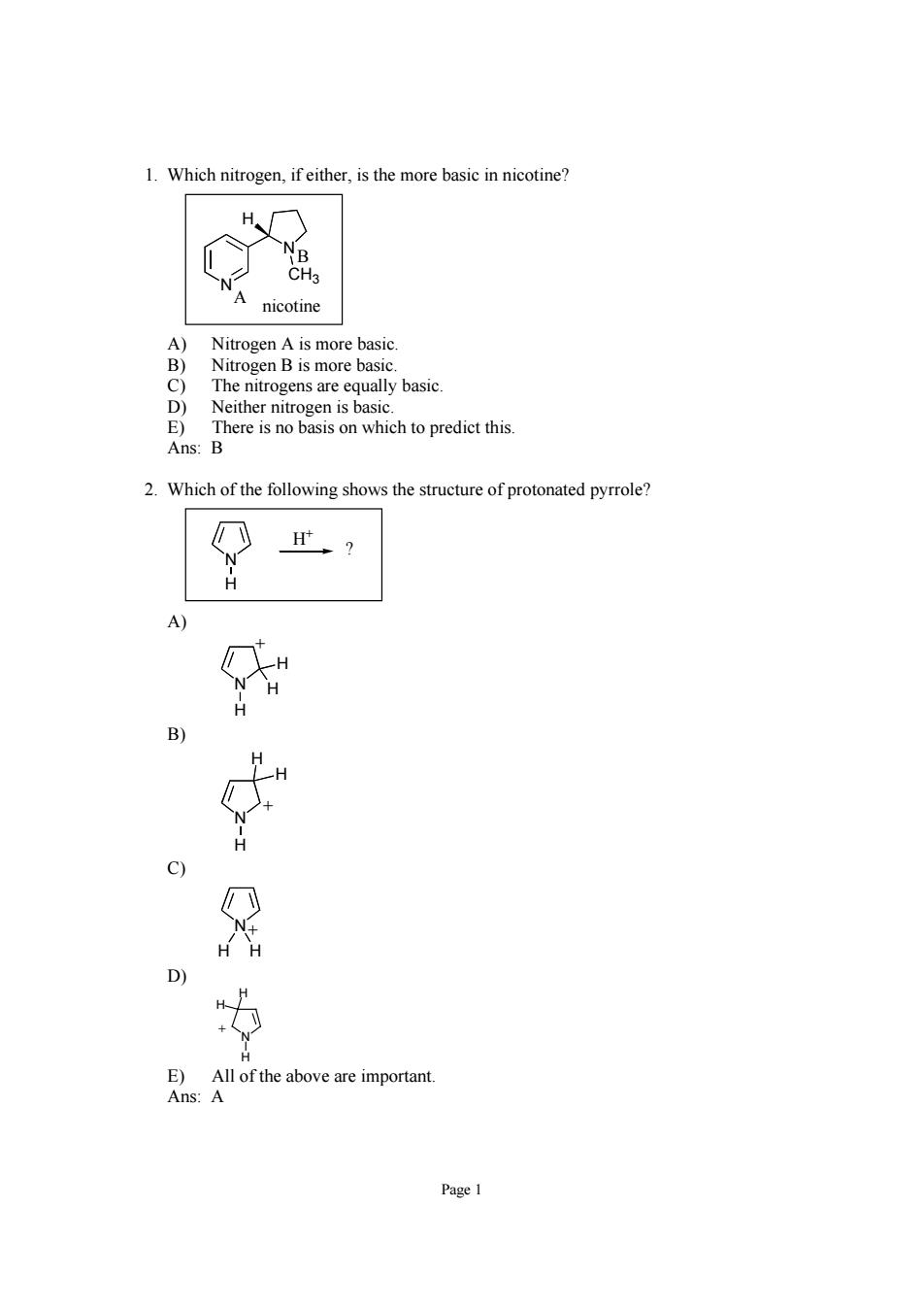
1.Which nitrogen,ifeither.is the more basic in nicotine H nicotine Nitrogen A is more basic Nitrogen B is more basic. mge to tt The Ans:B 2.Which of the following shows the structure of protonated pyrrole? C) E)All ofthe above are important. Ans:A
Page 1 1. Which nitrogen, if either, is the more basic in nicotine? N N CH3 H A B nicotine A) Nitrogen A is more basic. B) Nitrogen B is more basic. C) The nitrogens are equally basic. D) Neither nitrogen is basic. E) There is no basis on which to predict this. Ans: B 2. Which of the following shows the structure of protonated pyrrole? N H H+ ? A) N H H H + B) N H H H + C) N H H + D) N H + H H E) All of the above are important. Ans: A
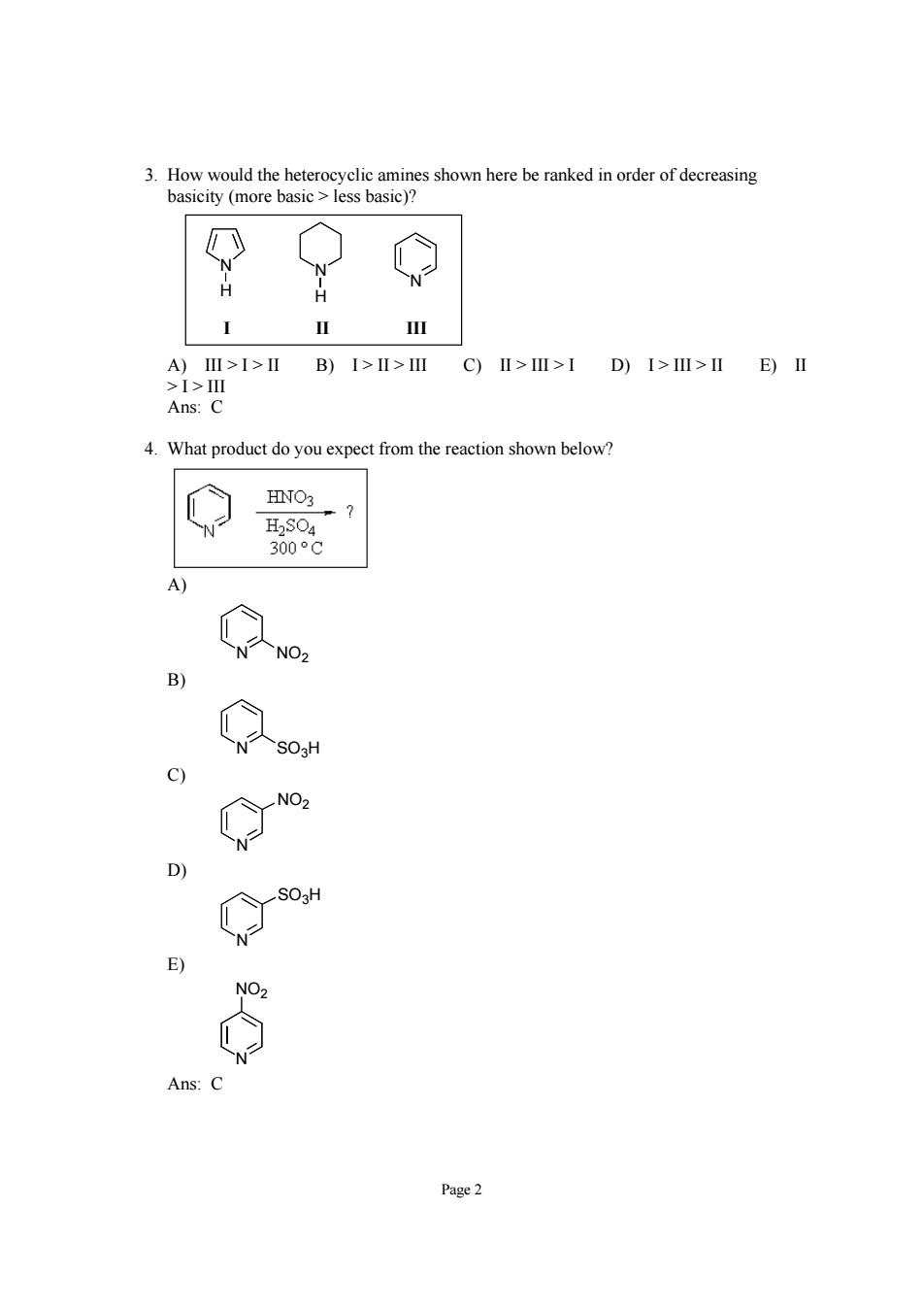
3.How would the heterocyclic amines shown here be ranked in order of decreasing basicity (more basic less basic)? A)Ⅲ>I>ⅡB)I>II>IC)Ⅱ>Ⅲ>IDI>Ⅲ>IIE)Ⅱ >1>Ⅲ Ans:C 4.What product do you expect from the reaction shown below? HNO3 NO -503 NO NO, Ans Page2
Page 2 3. How would the heterocyclic amines shown here be ranked in order of decreasing basicity (more basic > less basic)? N N N H H I II III A) III > I > II B) I > II > III C) II > III > I D) I > III > II E) II > I > III Ans: C 4. What product do you expect from the reaction shown below? A) N NO2 B) N SO3H C) N NO2 D) N SO3H E) N NO2 Ans: C
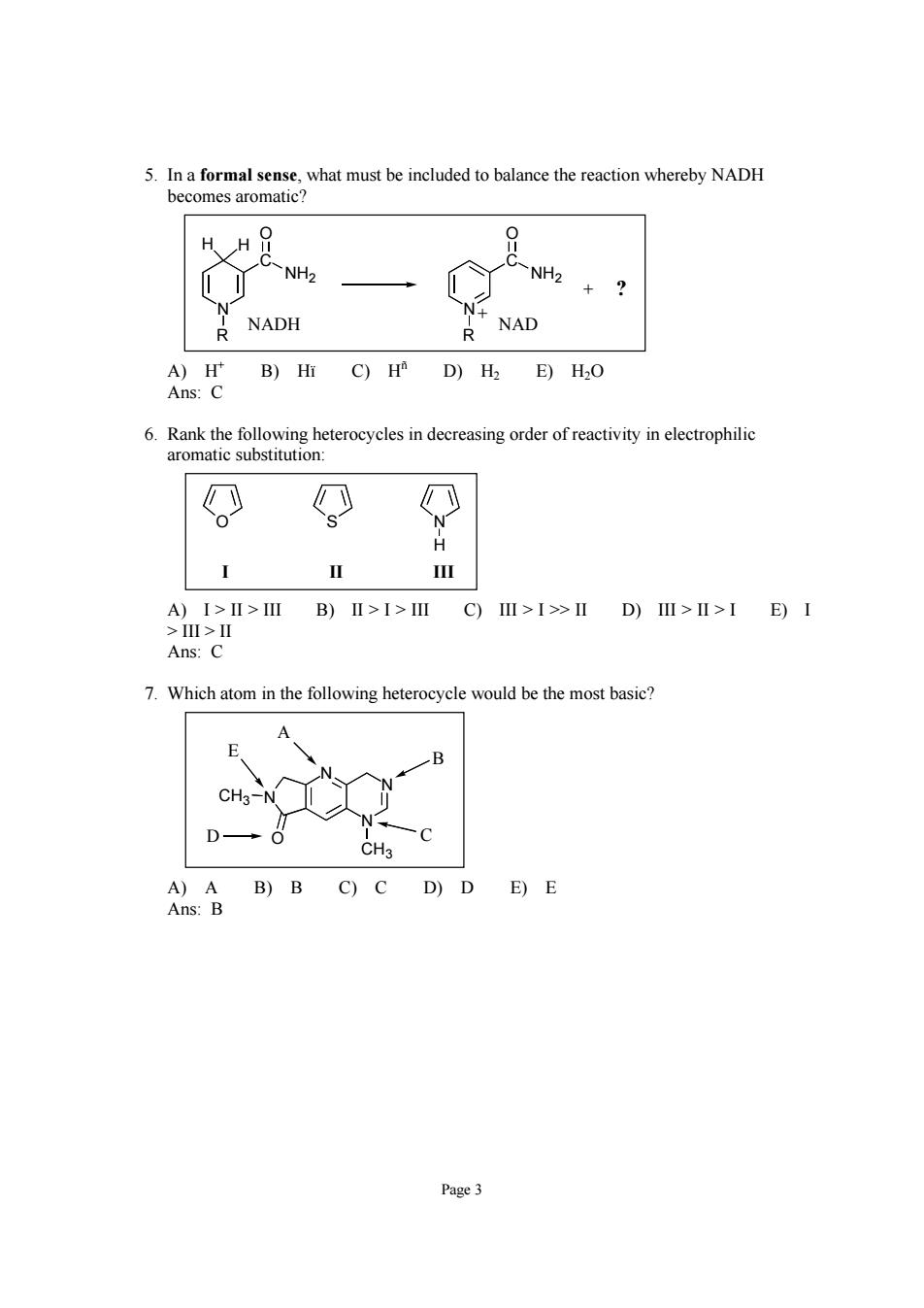
NH2+? R NADH R NAD 6.Rank the following heterocycles in decreasing order of reactivity in electrophilic aromatic substitution: O O H A)I>Ⅱ>ⅢB)Ⅱ>I>ⅢC)Ⅲ>I>ⅡD)I>I>IE)I >>Ⅱ Ans: 7.Which atom in the following heterocycle would be the most basic? g A)A B)B C)C D)D E)E Ans:B Page3
Page 3 5. In a formal sense, what must be included to balance the reaction whereby NADH becomes aromatic? N R C O NH2 N + R C O NH2 H H + ? NADH NAD A) H+ B) Hï C) Hñ D) H2 E) H2O Ans: C 6. Rank the following heterocycles in decreasing order of reactivity in electrophilic aromatic substitution: OSN I II III H A) I > II > III B) II > I > III C) III > I >> II D) III > II > I E) I > III > II Ans: C 7. Which atom in the following heterocycle would be the most basic? N CH3 N O N N CH3 A B D C E A) A B) B C) C D) D E) E Ans: B
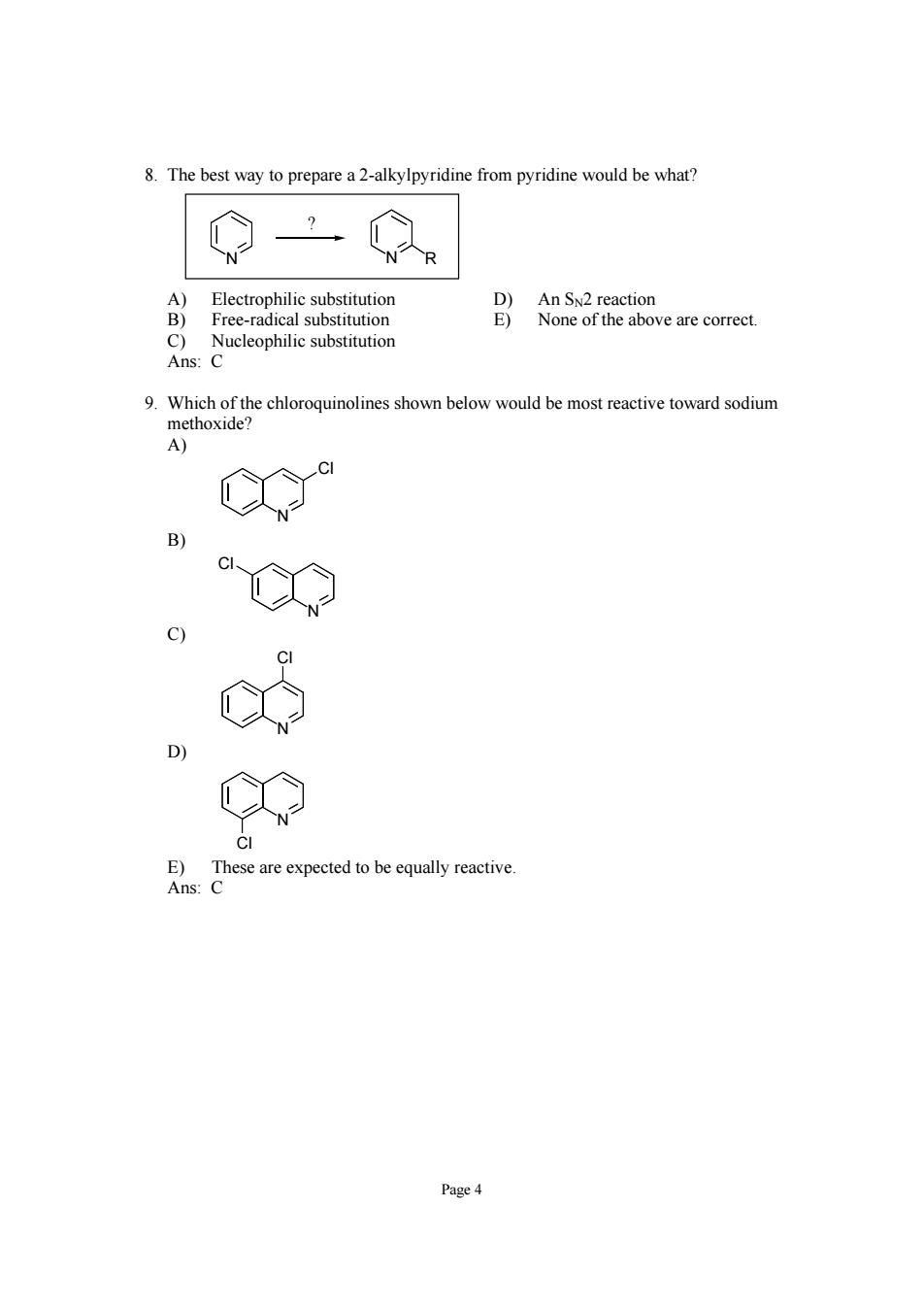
8.The best way to prepare a2-alkylpyridine from pyridine would be what? N人R 含} D)An Sx2 reaction Free-radical substitution None of the above are correct. Nucleophilic substitution 9.Which of the chloroquinolines shown below would be most reactive toward sodium methoxide? A) D E)These are expected to be equally reactive. Ans:C Page4
Page 4 8. The best way to prepare a 2-alkylpyridine from pyridine would be what? N N R ? A) Electrophilic substitution D) An SN2 reaction B) Free-radical substitution E) None of the above are correct. C) Nucleophilic substitution Ans: C 9. Which of the chloroquinolines shown below would be most reactive toward sodium methoxide? A) N Cl B) N Cl C) N Cl D) N Cl E) These are expected to be equally reactive. Ans: C

10.Which of the following would be the most reactive diene ina Diels-Alder reaction 名 Q c) C B)or undero Dicl-Alder reaction Page5
Page 5 10. Which of the following would be the most reactive diene in a Diels-Alder reaction? A) N H B) O C) N D) These are equally reactive. E) None of these undergo Diels-Alder reactions. Ans: B