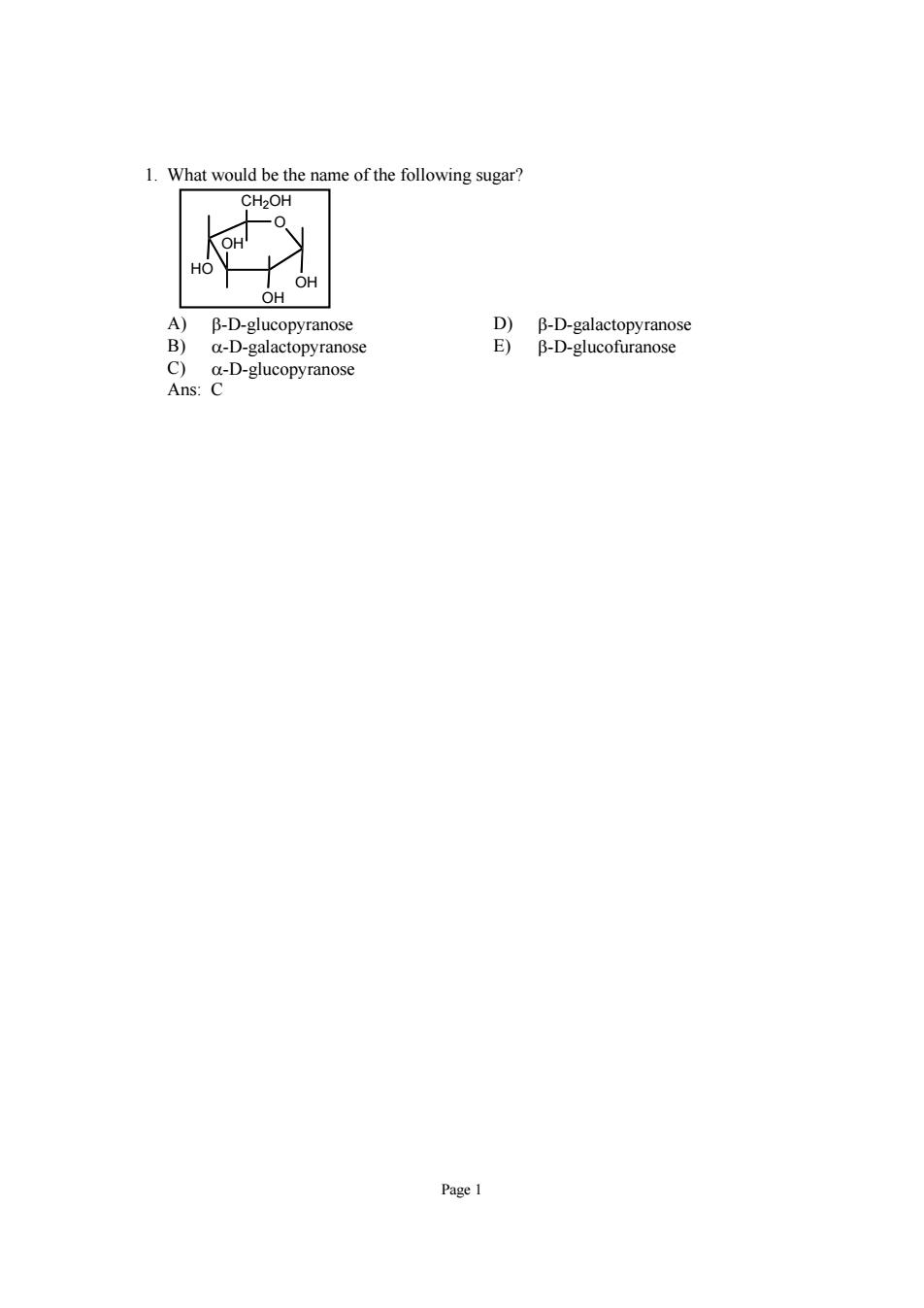
What would be the name of the following sugar CH2OH H A)B-D-glucopyranose D)B-D-galactopyranose B)a-D-galactopyranose E)B-D-glucofuranose C)a-D-glucopyranose Ans:C
Page 1 1. What would be the name of the following sugar? O OH OH OH HO CH2OH A) β-D-glucopyranose D) β-D-galactopyranose B) α-D-galactopyranose E) β-D-glucofuranose C) α-D-glucopyranose Ans: C

2.Which compound would result from the following reaction? CHO HO- HO- -H Br2.H2O H0- pH5-6 H -OH CH2OH HO- H HO- HO- CH2OH hO- HO- -H H COH HO- HO- -H H0- D) E) Page2
Page 2 2. Which compound would result from the following reaction? CHO CH2OH HO H HO H HO H H OH Br2, H2O pH 5-6 ? A) CO2H CH2OH HO H HO H HO H H OH B) CHO CO2H HO H HO H HO H H OH C) CO2H CO2H HO H HO H HO H H OH D) CH2OH CH2OH O HO H HO H H OH E)

Ans:A Page3
Page 3 CH2OH CH2OH HO H HO H HO H H OH Ans: A

3.Which one of the following could not rotate plane polarized light? A) - HO T0 CH-OH Ans. Page 4
Page 4 3. Which one of the following could not rotate plane polarized light? A) CH2OH C C C C CH2OH OH H OH OH H HO H H B) CHO C C C C CH2OH OH H H H OH HO HO H C) CO2H C C C C CH2OH OH H OH H OH H HO H D) CO2H C C C C CO2H OH H H HO HO H OH H E) CHO C C C CH2OH OH OH H OH H H Ans: D

seawater Bromine i gbtheoxidaionofb Addition to n fatt tyrosine,a phenol) 9 Oxidation of aldoses to aldonic acids(e.g,CHO to CO2H) a-bromination of fatty acids(e.g.,RCH2CO2H-?RCHBrCO2H) E) None of these reactions could occur in living systems Ans:D Wfthe reactions nstw would be catalyzed 5 a glycosidase enzyme? 6-phosphate ruto6-phosphate sucrose invert sugar C) cellulose glucose 9 amylose glucose E)glucose 6-phosphate- glucose phosphate ion Ans:B 6.What would be the name of the following sugar structure? ho HO OH OH a-D-galactopyranose D)a-D-glucopyranose B-D-glucopyranose E)B-D-galactofuranose C) B-D-galactopyranose Ans:B 7.What would be the name of the following sugar? CHO H HO- -H HO CH2OH D-glucose B)L-glucose C)L-galactose D)L-mannose E) Ans: Page 5
Page 5 4. Bromine is a dense, deep red-brown liquid obtained by the oxidation of bromide ion in seawater. Bromine is very toxic. Which of the following reactions of bromine with organic materials would be least likely in a biological system (would not occur)? A) Addition to non-aromatic carbon-carbon double bonds (e.g., in fatty acids) B) Electrophillic aromatic substitution of very activated aromatic rings (e.g., in tyrosine, a phenol) C) Oxidation of aldoses to aldonic acids (e.g., CHO to CO2H) D) α-bromination of fatty acids (e.g., RCH2CO2H →?RCHBrCO2H) E) None of these reactions could occur in living systems Ans: D 5. Which of the reactions listed below would be catalyzed by a glycosidase enzyme? A) glucose 6-phosphate fructose 6-phosphate B) sucrose invert sugar C) cellulose glucose D) amylose glucose E) glucose 6-phosphate glucose + phosphate ion Ans: B 6. What would be the name of the following sugar structure? O OH OH HO HO HOH2C A) α-D-galactopyranose D) α-D-glucopyranose B) β-D-glucopyranose E) β-D-galactofuranose C) β-D-galactopyranose Ans: B 7. What would be the name of the following sugar? CHO HO H H OH HO H HO H CH2OH A) D-glucose B) L-glucose C) L-galactose D) L-mannose E) D-mannose Ans: B