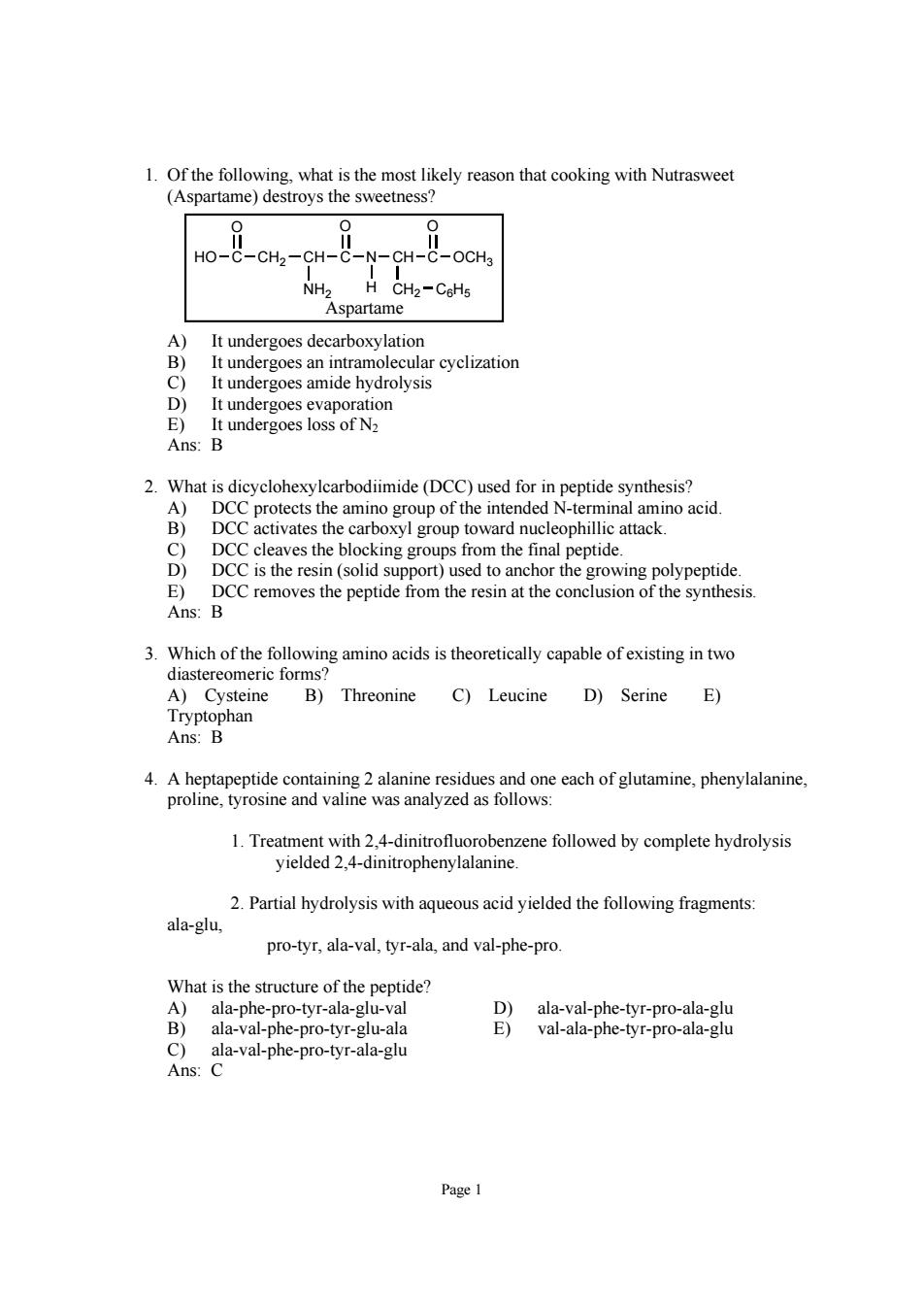
1.Of the following.what is the most likely reason that cooking with Nutrasweet (Aspartame)destroys the sweetness? HO-C-CH2-CH-C CH-C-OCHa NH2 CHz-CeHs Aspartame It undergoes decarboxylatior t un ergoes an intramol rcyclization D ergoes amide E)It undergoes loss of N Ans:B 2.What is dicyclohexylearbodiimide(DCC)used for in peptide synthesis? DCC protects the oxyl group towar eophill es the n the id ng gro ntia E)DCC nthesis Ans:B 3.Which of the following amino acids is theoretically capable of existing in two diastereomeric forms A)Cysteine B)Threonine C)Leucine D)Serine E) 4.A heptapeptide containing 2 alanine residues and one each of glutamine,phenylalanine, proline,tyrosine and valine was analyzed as follows: 1.Treatment with 2,4-dinitrofluorobenzene followed by complete hydrolysis yielded 2.4-dinitrophenylalanine. 2.Partial hydrolysis with aqueousacid yielded the following fragments ala-glu pro-tyr,ala-val,tyr-ala,and val-phe-pro What is the structure of the peptide? D)ala-val-phe-tyr-pro-ala-glu ala-val-phe-pro-tyr-glu-ala val-ala-phe-tyr-pro-ala-glu
Page 1 1. Of the following, what is the most likely reason that cooking with Nutrasweet (Aspartame) destroys the sweetness? HO C O CH2 CH NH2 C O N CH H CH2 C6H5 C O OCH3 Aspartame A) It undergoes decarboxylation B) It undergoes an intramolecular cyclization C) It undergoes amide hydrolysis D) It undergoes evaporation E) It undergoes loss of N2 Ans: B 2. What is dicyclohexylcarbodiimide (DCC) used for in peptide synthesis? A) DCC protects the amino group of the intended N-terminal amino acid. B) DCC activates the carboxyl group toward nucleophillic attack. C) DCC cleaves the blocking groups from the final peptide. D) DCC is the resin (solid support) used to anchor the growing polypeptide. E) DCC removes the peptide from the resin at the conclusion of the synthesis. Ans: B 3. Which of the following amino acids is theoretically capable of existing in two diastereomeric forms? A) Cysteine B) Threonine C) Leucine D) Serine E) Tryptophan Ans: B 4. A heptapeptide containing 2 alanine residues and one each of glutamine, phenylalanine, proline, tyrosine and valine was analyzed as follows: 1. Treatment with 2,4-dinitrofluorobenzene followed by complete hydrolysis yielded 2,4-dinitrophenylalanine. 2. Partial hydrolysis with aqueous acid yielded the following fragments: ala-glu, pro-tyr, ala-val, tyr-ala, and val-phe-pro. What is the structure of the peptide? A) ala-phe-pro-tyr-ala-glu-val D) ala-val-phe-tyr-pro-ala-glu B) ala-val-phe-pro-tyr-glu-ala E) val-ala-phe-tyr-pro-ala-glu C) ala-val-phe-pro-tyr-ala-glu Ans: C

5.Why is the following not a good two step route for the preparation of pure L-alanine? catalytic excess CH:CH2CO2H Br2 PBr2 NH The bromin a secondary brom Only the CHa group is brominated The product will he racemie E)Propanamide would be the major product. Ans:D 6.Disulfide bonds in proteins B刷 (RSH) h f proteins mk e in e can be broken n E) All of these are true. Ans:E 7.Which amino acid does not form ac B) Lysine E)Tryptophan 8.What material can be used repetitively (on a given sample)to sequence a polypeptide from the N-terminal end? A)Chymotrypsin D)Edman reagent B)Sanger reagent E)Carboxypeptidase Ans:Drypsin 9.How many tripeptides containing one residue each ofLalanine,L-valine,and glycine ssibl B)3C)4D)6E)9 Ans:D Page2
Page 2 5. Why is the following not a good two step route for the preparation of pure L-alanine? CH3CH2CO2H + Br2 catalytic PBr3 excess NH3 A) The bromination is not selective (the CH3 and CH2 groups are both brominated). B) Ammonia is not sufficiently nucleophillic to react with a secondary bromide. C) Only the CH3 group is brominated. D) The product will be racemic. E) Propanamide would be the major product. Ans: D 6. Disulfide bonds in proteins A) result from oxidation of thiols (RSH). B) function to help maintain the shape of proteins. C) can be broken by reduction reactions. D) link two cysteine amino acid residues. E) All of these are true. Ans: E 7. Which amino acid does not form a characteristic blue color with ninhydrin? A) Cysteine B) Lysine C) Proline D) Valine E) Tryptophan Ans: C 8. What material can be used repetitively (on a given sample) to sequence a polypeptide from the N-terminal end? A) Chymotrypsin D) Edman reagent B) Sanger reagent E) Carboxypeptidase C) Trypsin Ans: D 9. How many tripeptides containing one residue each of L-alanine, L-valine, and glycine are possible? A) 2 B) 3 C) 4 D) 6 E) 9 Ans: D
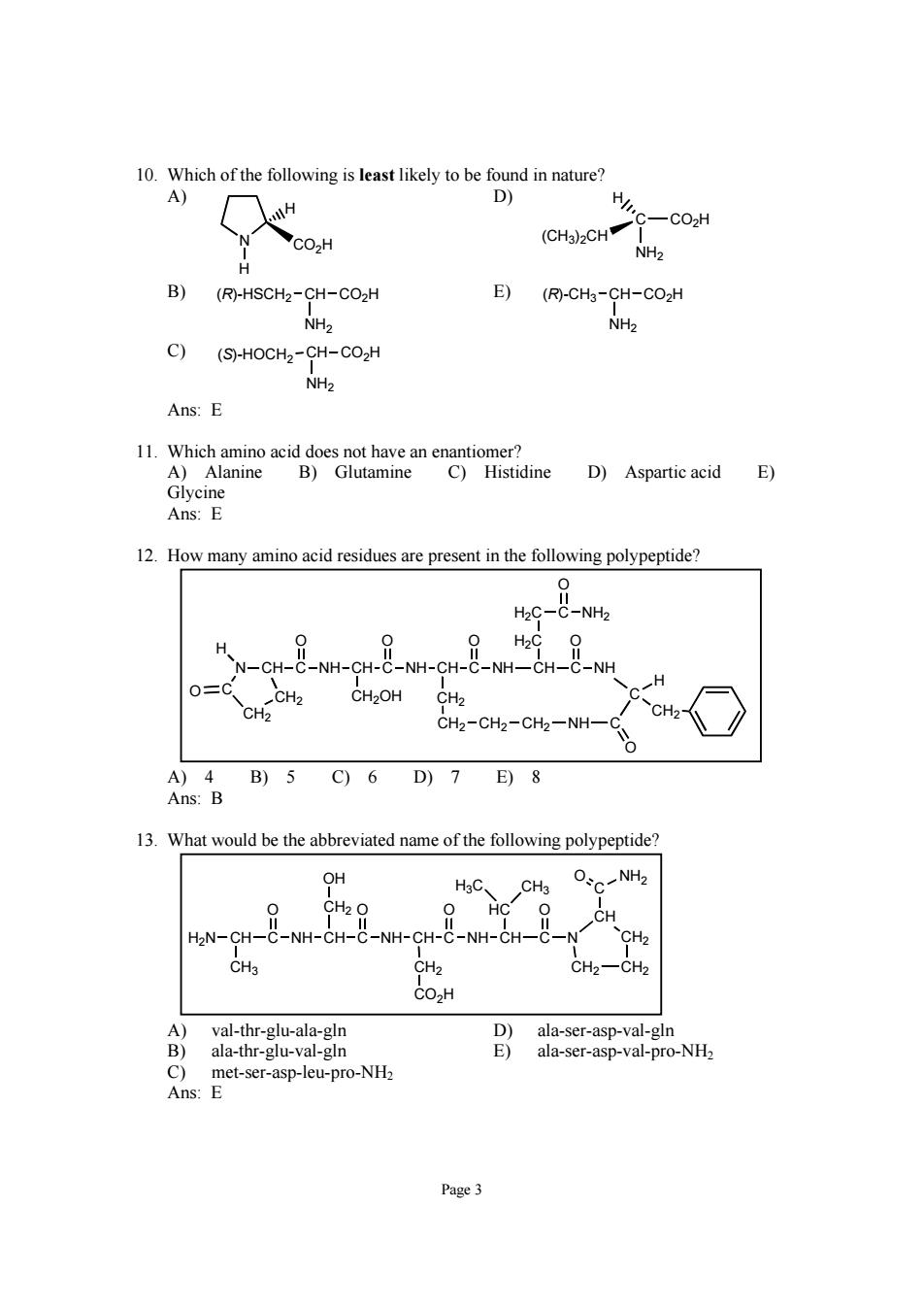
10.Which of the following is least likely to be found in nature? A) D) H H C-CO2H CO2H (CHa)2CH NH> B) (R)-HSCH2-CH-CO2H E)(风-CH-CH-C02H NH2 NH2 C)(S)-HOCH2-CH-CO2H NH2 Ans:E 1. D)Aspartic acid E) Ans:E 12.How many amino acid residues are present in the following polypeptide? H2C-C-NH2 H O=C H CH-CH CH2OH CH2 CH2-CH2-CH2-NH- c、 A)4 B)5C)6D)7E)8 Ans:B 13.What would be the abbreviated name of the following polypeptide? OH CHs 0 HC 0、 H2N-CH-C NH. NH. CH-C-NH-CH-C CH2 CH2一CH2 CO2H A)val-thr-glu-ala-gln ala-ser-asp-val-gln ap-leu-pro-NH. ala-thr-glu-val-gln 91 ala-ser-asp-val-pro-NHz Page3
Page 3 10. Which of the following is least likely to be found in nature? A) N CO2H H H D) (CH3)2CH C H CO2H NH2 B) (R)-HSCH2 CH CO2H NH2 E) (R)-CH3 CH CO2H NH2 C) (S)-HOCH2 CH CO2H NH2 Ans: E 11. Which amino acid does not have an enantiomer? A) Alanine B) Glutamine C) Histidine D) Aspartic acid E) Glycine Ans: E 12. How many amino acid residues are present in the following polypeptide? N CH C NH CH C NH CH C NH CH OOO O C NH C CH2OH CH2 CH2 CH2 CH2 NH C O C H CH2 H2C H2C C O NH2 CH2 CH2 H O A) 4 B) 5 C) 6 D) 7 E) 8 Ans: B 13. What would be the abbreviated name of the following polypeptide? H2N CH C NH CH C NH CH C NH CH O OO O C N CH2 CO2H CH2 OH CH CH2 CH3 CH2 CH2 C O NH2 HC H3C CH3 A) val-thr-glu-ala-gln D) ala-ser-asp-val-gln B) ala-thr-glu-val-gln E) ala-ser-asp-val-pro-NH2 C) met-ser-asp-leu-pro-NH2 Ans: E
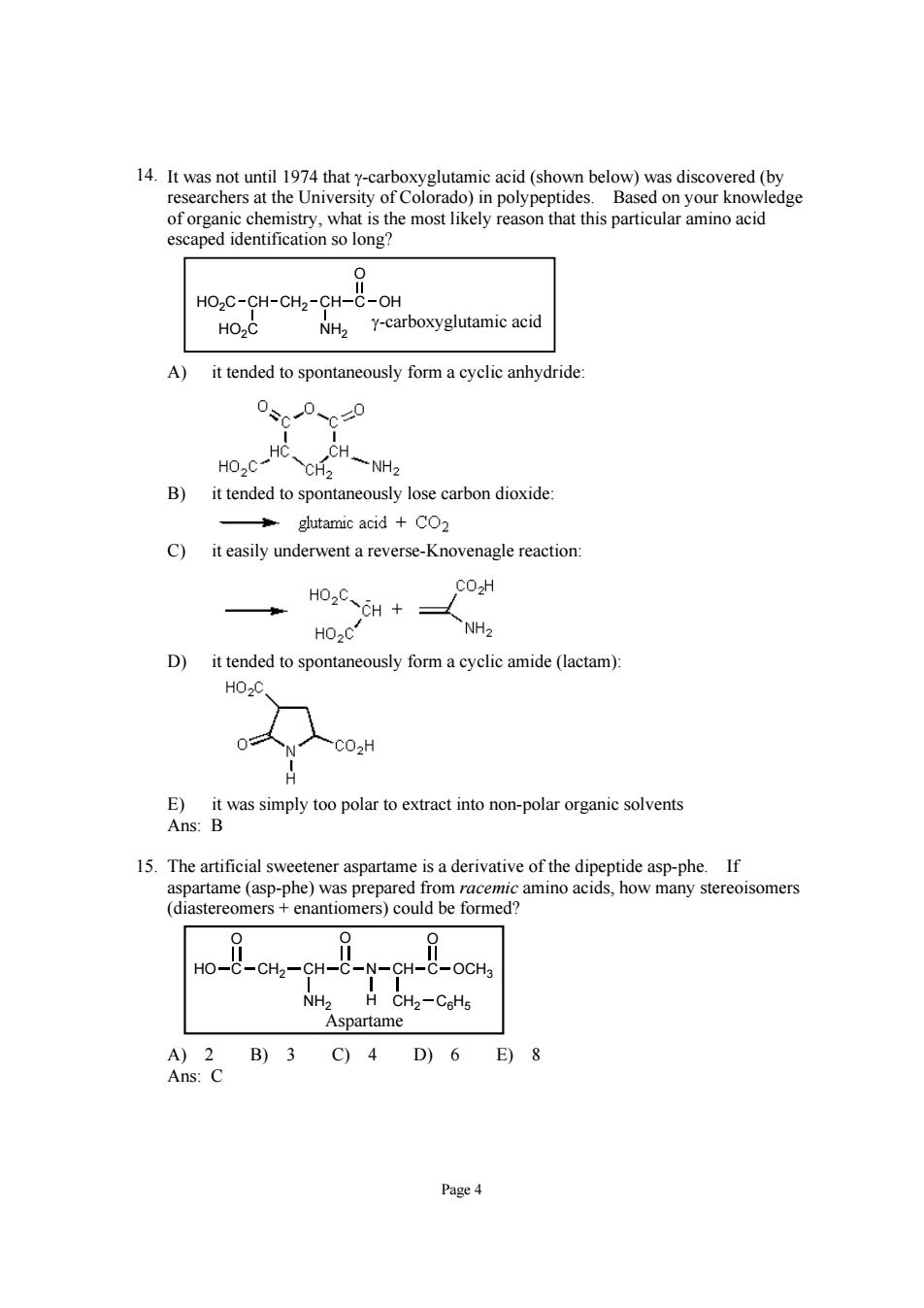
researchers ledge cation so long HO2C-CH-CH2-CH-C-OH HO2C NH2 Y-carboxyglutamic acid A) it tended to spontaneously form a cyclic anhydride 0、0、0 B)it tended to spontaneously lose carbon dioxide glutamic acid +CO2 C)it easily underwent a reverse-Knovenagle reaction HO.CCH+ CO2H HO2C D)it tended to spontaneously form a cyclic amide(lactam) HO.O 0 CO2H E)it was simply too polar to extract into non-polar organic solvents Ans:B 15.The artificial sweetener aspartame is a derivative of the dipeptide asp-phe.If aspartame(asp-phe)was prepared from racemic amino acids,how many stereoisomers (diastereomers enantiomers)could be formed? Ho--CH-CH- -C-OCHa Aspartame A)2B)3C)4D)6E)8 Ans:C Page4
Page 4 14. It was not until 1974 that γ-carboxyglutamic acid (shown below) was discovered (by researchers at the University of Colorado) in polypeptides. Based on your knowledge of organic chemistry, what is the most likely reason that this particular amino acid escaped identification so long? CH CH2 CH C OH O HO2C HO2C NH2 γ-carboxyglutamic acid A) it tended to spontaneously form a cyclic anhydride: B) it tended to spontaneously lose carbon dioxide: C) it easily underwent a reverse-Knovenagle reaction: D) it tended to spontaneously form a cyclic amide (lactam): E) it was simply too polar to extract into non-polar organic solvents Ans: B 15. The artificial sweetener aspartame is a derivative of the dipeptide asp-phe. If aspartame (asp-phe) was prepared from racemic amino acids, how many stereoisomers (diastereomers + enantiomers) could be formed? HO C O CH2 CH NH2 C O N CH H CH2 C6H5 C O OCH3 Aspartame A) 2 B) 3 C) 4 D) 6 E) 8 Ans: C

16.Chymotrypsin catalyzes hydrolysis of peptide bonds where? residue in question 0 0 NU-CL NH-etc "carboxy end" "amino end" A)At the carboxy end of residues with basic side chains At the of re dues w ith aromatic es with aromat Ans:C 17.The primary structure of a protein refers to the A) sequence of amino acids. one degree of 18.Which of the following amino acids would have the highest isoelectric point? A)Lysine B)Glutamine C)Aspartic acid D)Alanine E) Tryptophan Ans:A 19.Nearly all a A Lysine B)Histidine C)Tyrosine D)Cysteine E)Proline Ans:E Page5
Page 5 16. Chymotrypsin catalyzes hydrolysis of peptide bonds where? etc. C NH CH C NH etc. O O R residue in question "amino end" "carboxy end" A) At the carboxy end of residues with basic side chains B) At the amino end of residues containing aliphatic residues C) At the carboxy end of residues with aromatic side chains D) At the amino end of residues with aromatic side chains E) At the carboxy end of methionine residues Ans: C 17. The primary structure of a protein refers to the A) sequence of amino acids. B) overall 3-dimensional shape. C) localized shape of the backbone. D) degree of aggregation with other proteins. E) structure of the active site. Ans: A 18. Which of the following amino acids would have the highest isoelectric point? A) Lysine B) Glutamine C) Aspartic acid D) Alanine E) Tryptophan Ans: A 19. Nearly all amino acids immediately release N2 gas upon diazotization (treatment with aqueous HCl and sodium nitrite). Which amino acid would not? A) Lysine B) Histidine C) Tyrosine D) Cysteine E) Proline Ans: E