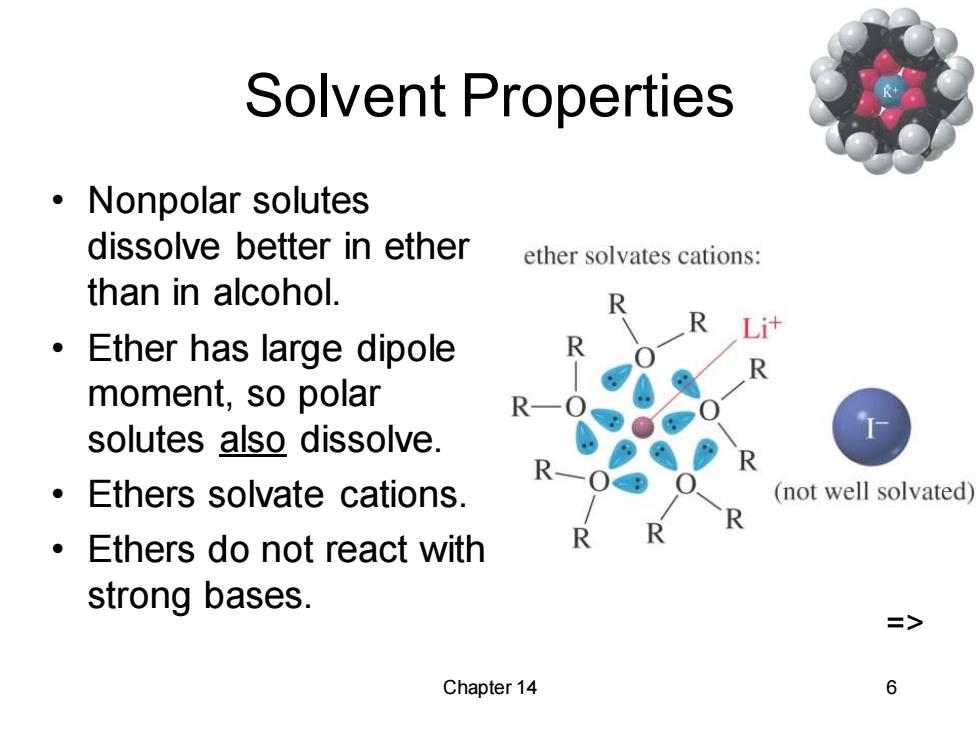
Solvent Properties ·Nonpolar solutes dissolve better in ether ether solvates cations: than in alcohol. 0 Ether has large dipole moment,so polar R solutes also dissolve. Ethers solvate cations. (not well solvated) Ethers do not react with strong bases. => Chapter 14 6
Chapter 14 6 Solvent Properties • Nonpolar solutes dissolve better in ether than in alcohol. • Ether has large dipole moment, so polar solutes also dissolve. • Ethers solvate cations. • Ethers do not react with strong bases. =>
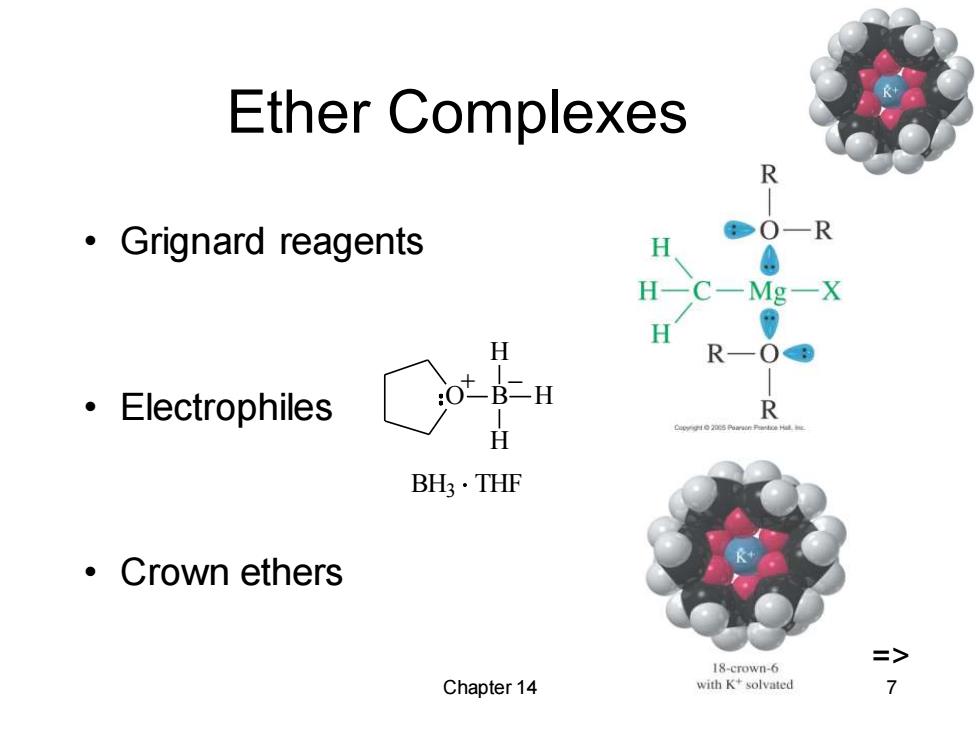
Ether Complexes R ·Grignard reagents O-R H H-C一Mg-X H H R—O8 ·Electrophiles :OtB二H R H BH3·THF ·Crown ethers 18-crown-6 Chapter 14 with K+solvated 7
Chapter 14 7 Ether Complexes • Grignard reagents • Electrophiles • Crown ethers O B H H H + _ BH3 THF =>
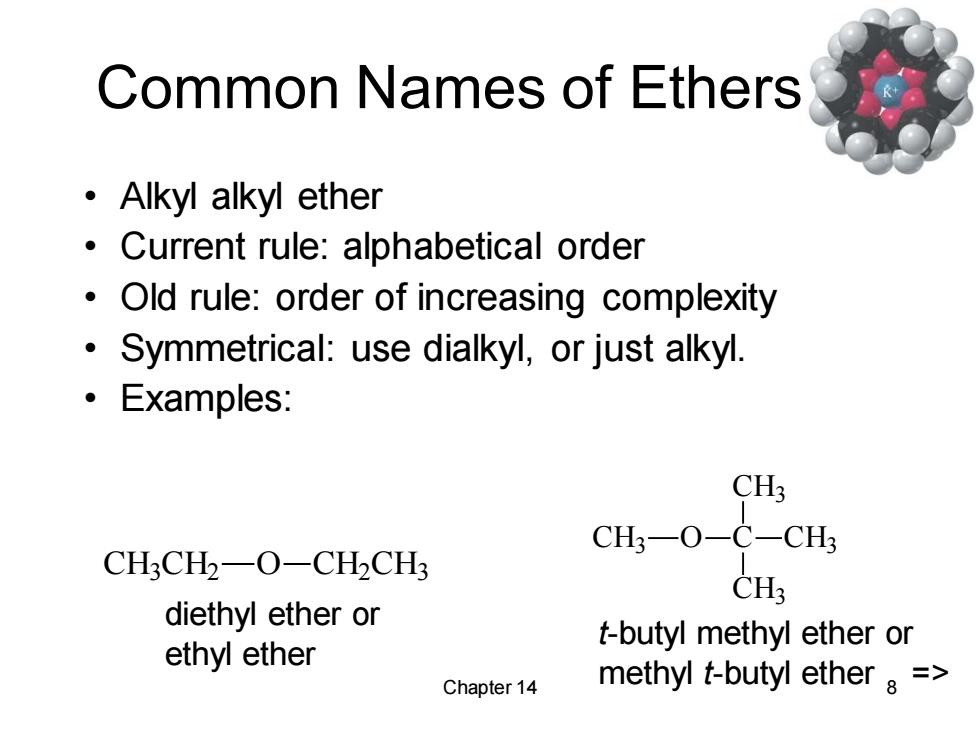
Common Names of Ethers ·Alkyl alkyl ether Current rule:alphabetical order Old rule:order of increasing complexity 。 Symmetrical:use dialkyl,or just alkyl. ·Examples: CH3 CH3一O-C-CH3 CH3CH2-O-CH2CH3 CH3 diethyl ether or t-butyl methyl ether or ethyl ether Chapter 14 methyl t-butyl ether a =
Chapter 14 8 Common Names of Ethers • Alkyl alkyl ether • Current rule: alphabetical order • Old rule: order of increasing complexity • Symmetrical: use dialkyl, or just alkyl. • Examples: CH3 CH2 O CH2 CH3 diethyl ether or ethyl ether CH3 O C CH3 CH3 CH3 t-butyl methyl ether or methyl t-butyl ether =>
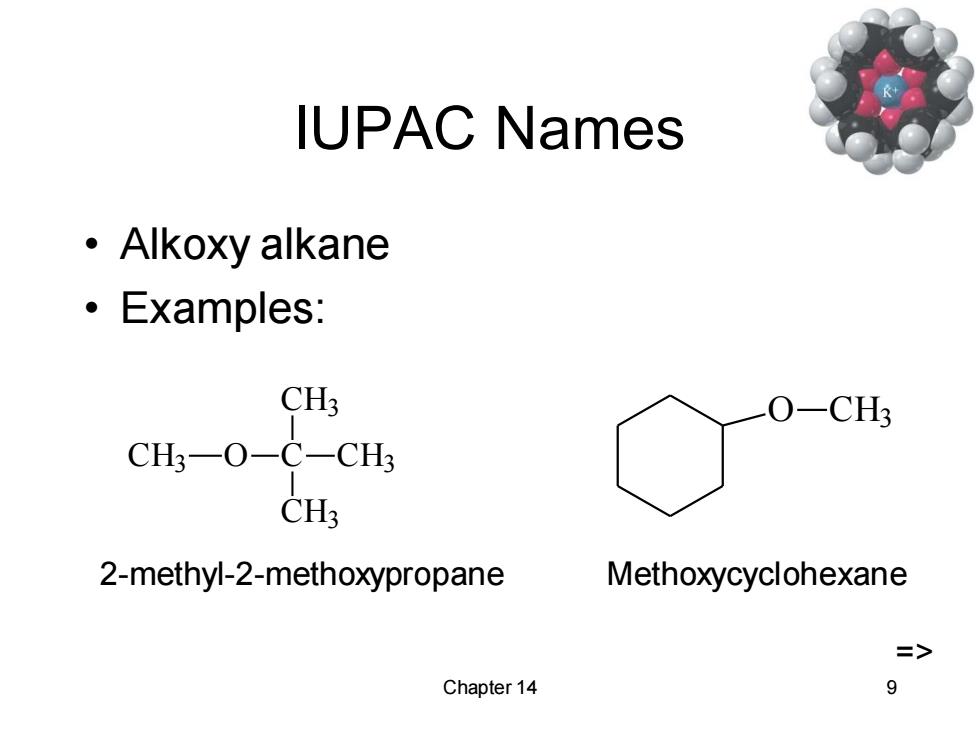
IUPAC Names ·Alkoxy alkane ·Examples: CH3 O-CH? CH3-O-C-CH3 CH3 2-methyl-2-methoxypropane Methoxycyclohexane => Chapter 14 9
Chapter 14 9 IUPAC Names • Alkoxy alkane • Examples: CH3 O C CH3 CH3 CH3 2-methyl-2-methoxypropane O CH3 Methoxycyclohexane =>
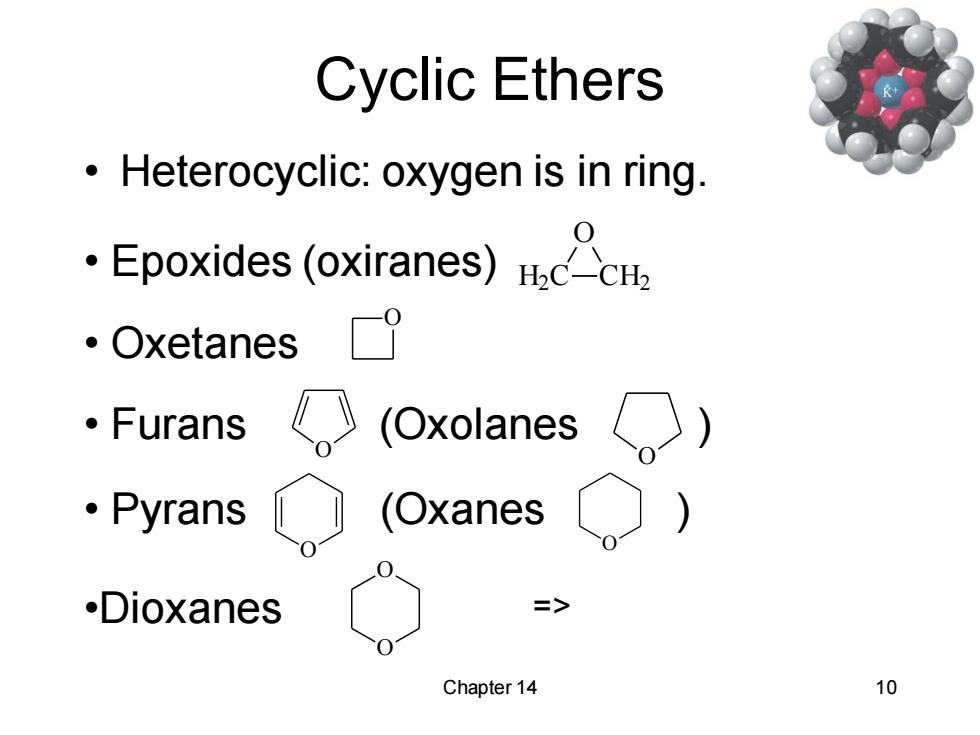
Cyclic Ethers Heterocyclic:oxygen is in ring Epoxides (oxiranes)c 。Oxetanes 。Furans 《(Oxolanes ·Pyrans (Oxanes .Dioxanes > Chapter 14 10
Chapter 14 10 Cyclic Ethers • Heterocyclic: oxygen is in ring. • Epoxides (oxiranes) H2C CH2 O • Oxetanes O • Furans (Oxolanes ) O O • Pyrans (Oxanes ) O O •Dioxanes O O =>