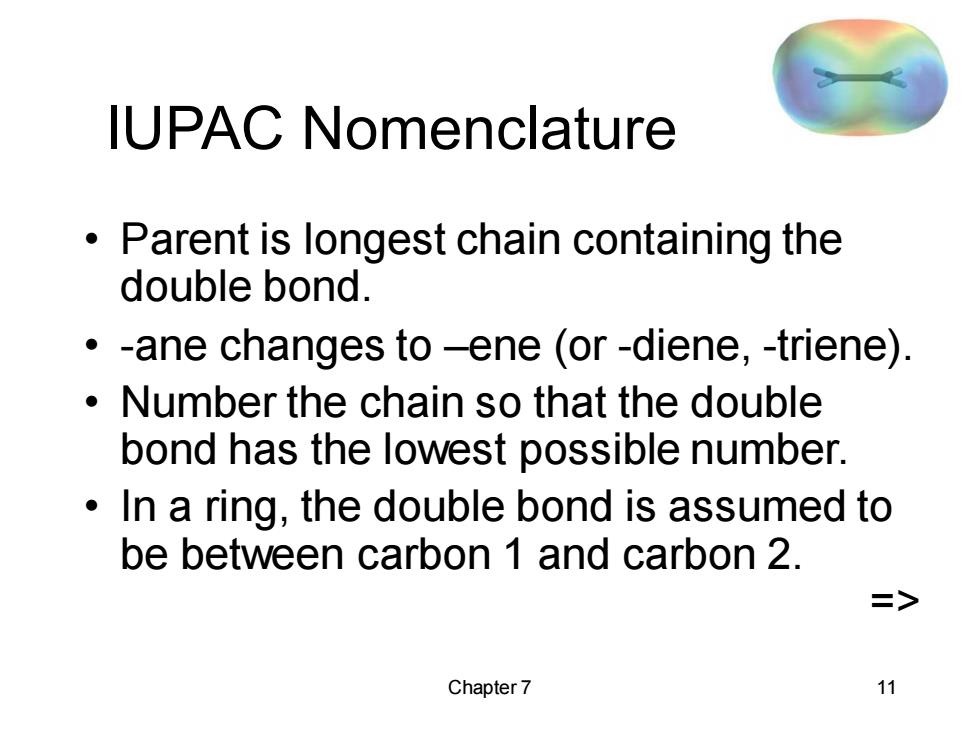
IUPAC Nomenclature Parent is longest chain containing the double bond. -ane changes to-ene (or-diene,-triene) Number the chain so that the double bond has the lowest possible number. In a ring,the double bond is assumed to be between carbon 1 and carbon 2. > Chapter 7 11
Chapter 7 11 IUPAC Nomenclature • Parent is longest chain containing the double bond. • -ane changes to –ene (or -diene, -triene). • Number the chain so that the double bond has the lowest possible number. • In a ring, the double bond is assumed to be between carbon 1 and carbon 2. =>
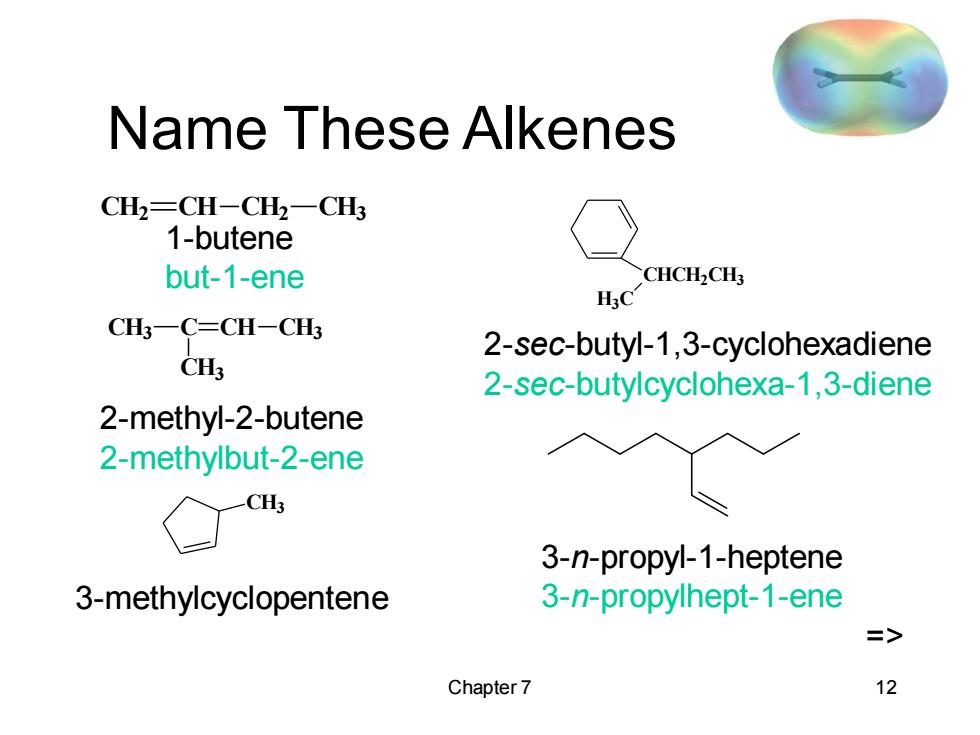
Name These Alkenes CH2=CH-CH2一CH 1-butene but-1-ene CHCHCH HaC CH一C=CH-CH 2-sec-butyl-1,3-cyclohexadiene CH3 2-sec-butylcyclohexa-1,3-diene 2-methyl-2-butene 2-methylbut-2-ene -CH3 3-n-propyl-1-heptene 3-methylcyclopentene 3-n-propylhept-1-ene => Chapter 7 12
Chapter 7 12 Name These Alkenes CH2 CH CH2 CH3 CH3 C CH3 CH CH3 CH3 CHCH2CH3 H3C 1-butene but-1-ene 2-methyl-2-butene 2-methylbut-2-ene 3-methylcyclopentene 2-sec-butyl-1,3-cyclohexadiene 2-sec-butylcyclohexa-1,3-diene 3-n-propyl-1-heptene 3-n-propylhept-1-ene =>
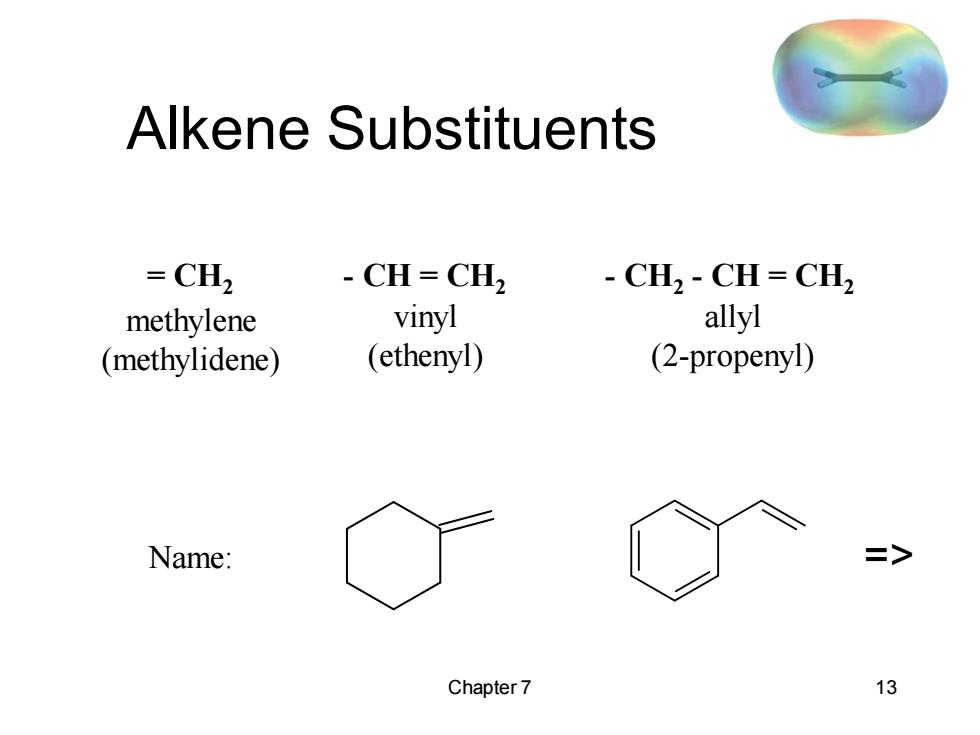
Alkene Substituents =CH2 -CH=CH2 CH,CH=CH2 methylene vinyl allyl (methylidene) (ethenyl) (2-propenyl) Name: > Chapter 7 13
Chapter 7 13 Alkene Substituents = CH2 methylene (methylidene) - CH = CH2 vinyl (ethenyl) - CH2 - CH = CH2 allyl (2-propenyl) Name: =>
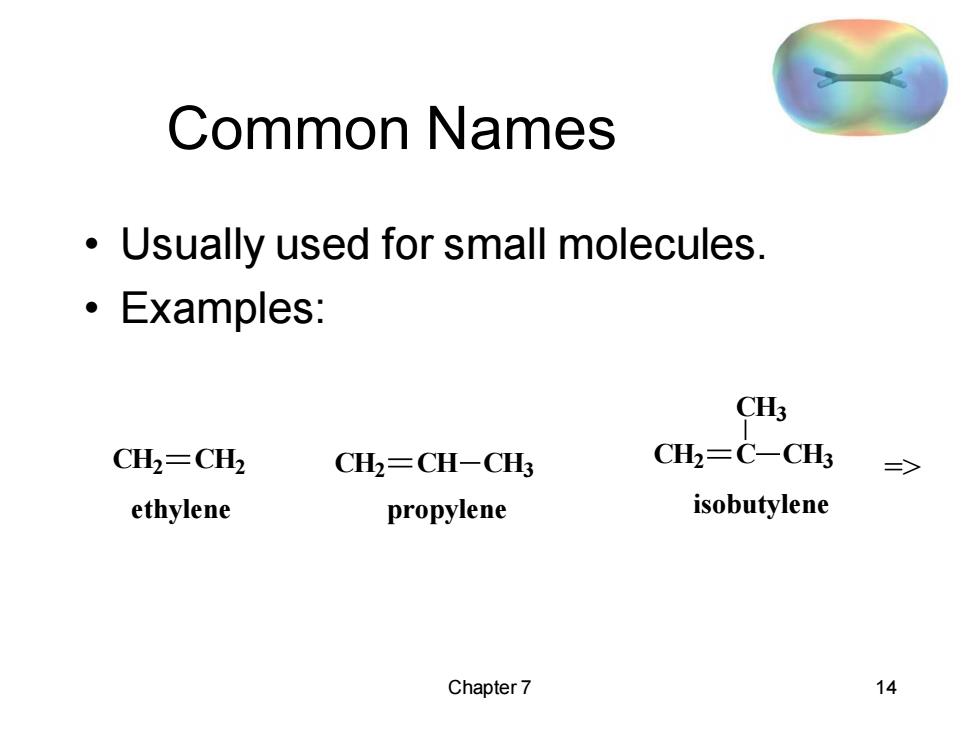
Common Names Usually used for small molecules. Examples: CH3 CH2=CH2 CH2=CH-CH3 CH2=C-CH3 => ethylene propylene isobutylene Chapter 7 14
Chapter 7 14 Common Names • Usually used for small molecules. • Examples: CH2 CH2 ethylene CH2 CH CH3 propylene CH2 C CH3 CH3 isobutylene =>