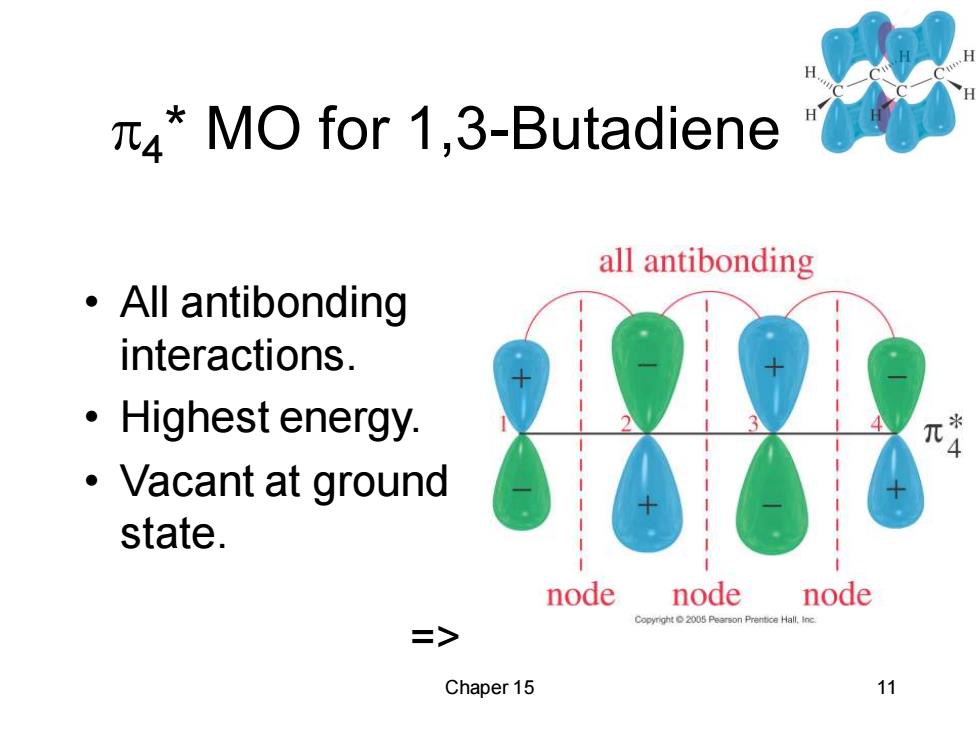
*MO for 1,3-Butadiene all antibonding ·All antibonding interactions. ·Highest energy. ·Vacant at ground state. node node node Copyright2005 Peerson Prentice Hall.Inc. => Chaper 15 11
Chaper 15 11 4 * MO for 1,3-Butadiene • All antibonding interactions. • Highest energy. • Vacant at ground state. =>
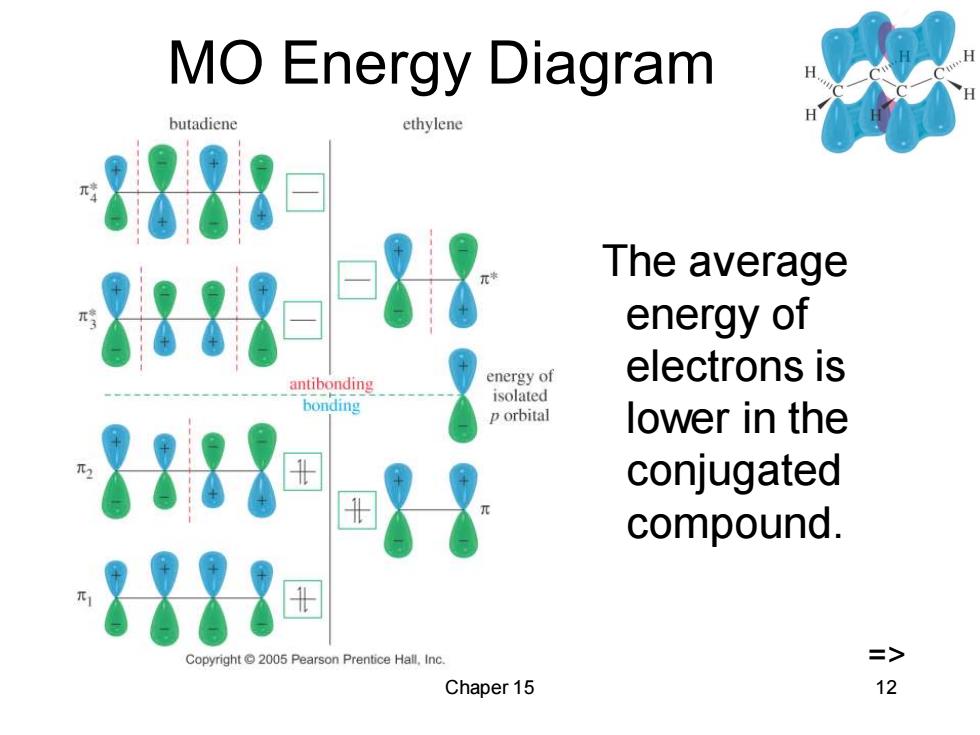
MO Energy Diagram butadiene ethylene 878 The average 881e energy of electrons is antibonding energy of bonding isolated orbital lower in the 888u conjugated compound. 873▣ Copyright 2005 Pearson Prentice Hall,Inc. => Chaper 15 12
Chaper 15 12 MO Energy Diagram The average energy of electrons is lower in the conjugated compound. =>

Conformations of 1,3-Butadiene s-trans conformer is more stable than the s-cis by 12 kJ/mol (2.8 kcal/mol). Easily interconvert at room temperature. s-trans S-cis Chaper 15 13
Chaper 15 13 Conformations of 1,3-Butadiene • s-trans conformer is more stable than the s-cis by 12 kJ/mol (2.8 kcal/mol). • Easily interconvert at room temperature. H H H H H H s-trans s-cis H H H H H H =>
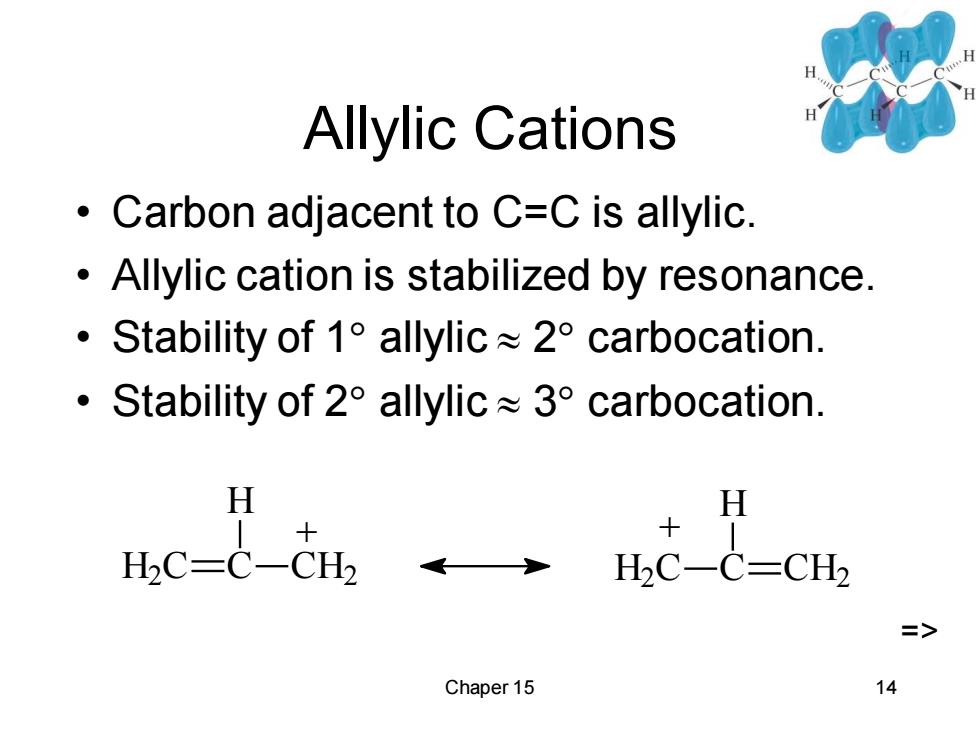
Allylic Cations Carbon adjacent to C=C is allylic Allylic cation is stabilized by resonance. ·Stability of1 allylic≈2t°carbocation. ·Stability of2°allylic≈3°carbocation. H H H2C-C-CH2 HC-C=CH2 => Chaper 15 14
Chaper 15 14 Allylic Cations • Carbon adjacent to C=C is allylic. • Allylic cation is stabilized by resonance. • Stability of 1 allylic 2 carbocation. • Stability of 2 allylic 3 carbocation. H2 C C H CH2 + H2 C C H CH2 + =>