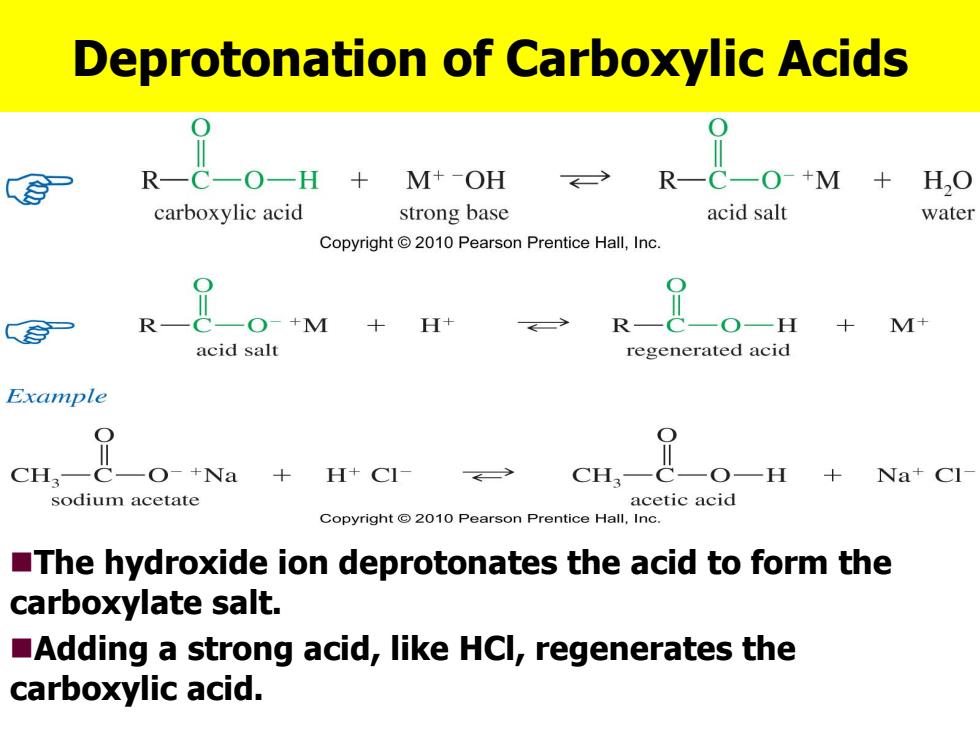
Deprotonation of Carboxylic Acids M--OH R-C-O-+M H,0 carboxylic acid strong base acid salt water Copyright 2010 Pearson Prentice Hall,Inc. ROM+H+ RO-H +M+ acid salt regenerated acid Example 是oNa+HC4 CH— CH, 0 +Na+Cl sodium acetate acetic acid Copyright @2010 Pearson Prentice Hall,Inc. The hydroxide ion deprotonates the acid to form the carboxylate salt. Adding a strong acid,like HCl,regenerates the carboxylic acid
Deprotonation of Carboxylic Acids The hydroxide ion deprotonates the acid to form the carboxylate salt. Adding a strong acid, like HCl, regenerates the carboxylic acid

Hydrolysis of Fats and Oils 0 CHz-0-c_ CH2-OH 0 CH-0-c_ OH/H,O CH-OH hydrolysis 0 CH2-0-c- CH2-OH 0-&M fat or oil glycerol fatty acid salts (soap) Copyright2010 Pearson Prentice Hall,Inc. The basic hydrolysis of fat and oils produces soap,known as saponification
Hydrolysis of Fats and Oils • The basic hydrolysis of fat and oils produces soap,known as saponification

Purifying or Extraction of Carboxylic Acids ether RCOOH other (1)remove ether phase organics R-COOH phase other (1)remove H2O phase (2)acidify with HCI(aq) (pure) organics (2)add dilute NaOH RCOO (3)add fresh ether (or NaHCO3) Na+ H2O salts, Na OH H+CI- etc. phase R—COH NaOH (aq) R-C-O-Na+ HCl (aq) R-C soluble in ether,but not in H2O soluble in H2O,but not in ether soluble in ether,but not in H2O A carboxylic acid is more soluble in the organic phase, but its salt is more soluble in the aqueous phase. Acid-base extractions can move the acid from the ether phase into the aqueous phase and back into the ether phase,leaving impurities behind
Purifying or Extraction of Carboxylic Acids A carboxylic acid is more soluble in the organic phase, but its salt is more soluble in the aqueous phase. Acid–base extractions can move the acid from the ether phase into the aqueous phase and back into the ether phase, leaving impurities behind

Some Important Acids Acetic acid is in vinegar and other foods, used industrially as solvent,catalyst,and reagent for synthesis. Fatty acids from fats and oils. Benzoic acid in drugs,preservatives. Adipic acid used to make nylon 66. 己二酸 Phthalic acid used to make polyesters. 邻苯二甲酸
Some Important Acids Acetic acid is in vinegar and other foods, used industrially as solvent, catalyst, and reagent for synthesis. Fatty acids from fats and oils. Benzoic acid in drugs, preservatives. Adipic acid used to make nylon 66. Phthalic acid used to make polyesters. 邻苯二甲酸 己二酸

Sec 2 PREPARATIONS OF CARBOXYLIC ACIDS Functional group transformations oPrimary alcohols and aldehydes are converted to carboxylic acids by oxidation. Acid chlorides,acid anhydrides,esters,and amides can be hydrolyzed to their parent carboxylic acids ■Bond cleavage Cleavage of an alkene with hot KMnO4 produces a carboxylic acid if there is a hydrogen on the double- bonded carbon. Cleavage of an alkyne with ozone or hot permanganate. ■C-C bond formation Grignard Carboxylation alkylating diethyl malonate
Sec 2 PREPARATIONS OF CARBOXYLIC ACIDS Functional group transformations Primary alcohols and aldehydes are converted to carboxylic acids by oxidation. Acid chlorides, acid anhydrides, esters, and amides can be hydrolyzed to their parent carboxylic acids Bond cleavage Cleavage of an alkene with hot KMnO4 produces a carboxylic acid if there is a hydrogen on the double- bonded carbon. Cleavage of an alkyne with ozone or hot permanganate. C-C bond formation Grignard Carboxylation alkylating diethyl malonate