
Acidity R-0-H+H,δ:→ R-03 +HO+ pKa兰16 (Ka=10-16) alcohol alkoxide 0 R-C-0-H+H,:← pK≡5 R- HO+ (K2≡105) acid carboxylate R—O- + H.O+ R-OH H2O H0+ R-COOH H2O stabilization 0 of carboxylate Copyright2005 Pearson Prentice Hall,Inc
Acidity
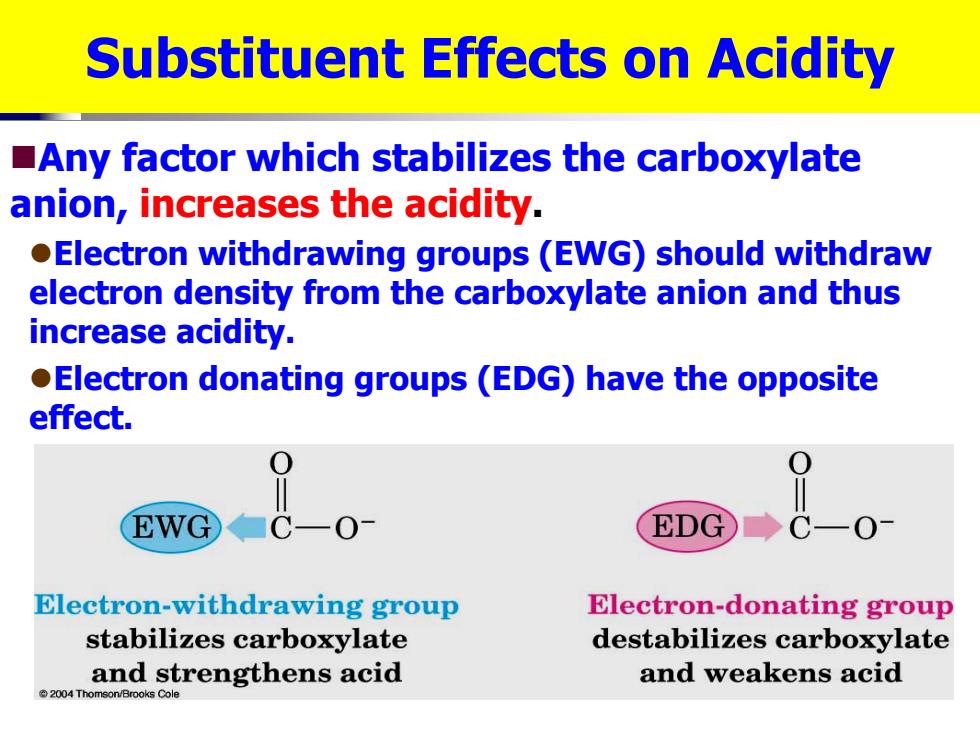
Substituent Effects on Acidity Any factor which stabilizes the carboxylate anion,increases the acidity. Electron withdrawing groups(EWG)should withdraw electron density from the carboxylate anion and thus increase acidity. Electron donating groups(EDG)have the opposite effect. EWG C-0 EDG ◆C一O Electron-withdrawing group Electron-donating group stabilizes carboxylate destabilizes carboxylate and strengthens acid and weakens acid 2004 Thomson/Brooks Cole
Substituent Effects on Acidity Any factor which stabilizes the carboxylate anion, increases the acidity . Electron withdrawing groups (EWG) should withdraw electron density from the carboxylate anion and thus increase acidity. Electron donating groups (EDG) have the opposite effect

Inductive effects诱导效应 Inductive effects are strongly dependent on distance.The effect of the halogen decreases as the distance increases. FCH2CO2H>CICH2CO2H>BrCH2CO2H>CH3CO2H pKa 2.59 2.87 2.90 4.76 CI3CCO,H>CI2CHCO,H>CICH2CO,H>CH3CO,H pKa 0.64 1.26 2.87 4.76 C02H> CO2H pKa 2.86 4.05 4.52
FCH2CO2H > ClCH2CO2H > BrCH2CO2H > CH3CO2H Cl3CCO2H > Cl2CHCO2H > ClCH2CO2H > CH3CO2H CO2H Cl CO2H Cl CO2H Cl > > pKa pKa pKa 2.59 2.87 2.90 4.76 0.64 1.26 2.87 4.76 2.86 4.05 4.52 Inductive effects 诱导效应 Inductive effects are strongly dependent on distance. The effect of the halogen decreases as the distance increases

Substituent Effects on Acidity LElectronegative substituents promote formation of the carboxylate ion Structure Ka pK FaCCO2H 0.59 0.23 Stronger acid FCH2CO2H 2.6×10-3 2.59 CICH2CO2H 1.4×10-3 2.85 BrCH2CO2H 2.1×10-3 2.68 ICH2CO2H 7.5×10-4 3.12 HCO2H 1.77×10-4 3.75 HOCH2CO2H 1.5×10-4 3.83 CcHCO2H 6.46×10-5 4.19 HC-CHCO,H 5.6×10-5 4.25 CH:CO2H 1.76×10-5 4.75 CH:CH2CO2H 1.34×10-5 4.87 Weaker acid CH CH2OH(ethanol)4 (10-16) (16) Value for ethanol is shown for reference 2004 Thomson/Brooks Cole
Substituent Effects on Acidity Electronegative substituents promote formation of the carboxylate ion
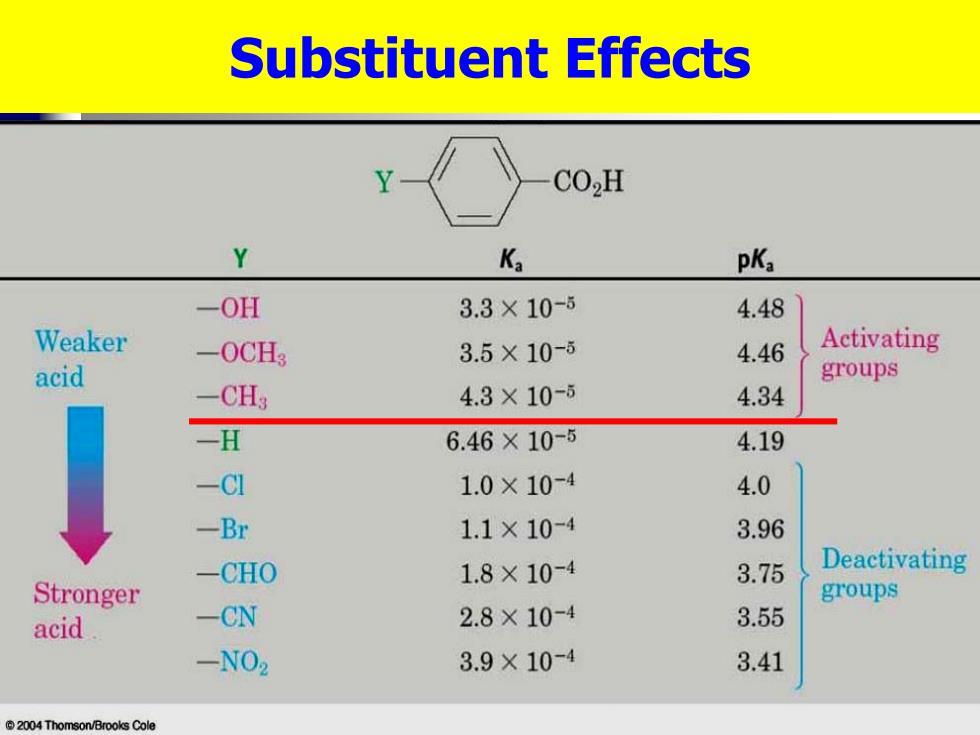
Substituent Effects CO,H Y Ka pKa -OH 3.3×10-6 4.48 Weaker -0CH3 3.5×10-ǒ 4.46 Activating acid groups -CH3 4.3×10-6 4.34 -Π 6.46×10-5 4.19 -C1 1.0×10-4 4.0 -Br 1.1×10-4 3.96 -CHO 1.8×10-4 3.75 Deactivating Stronger groups acid -CN 2.8×10-4 3.55 -NO2 3.9×10-4 3.41 2004 Thomson/Brooks Cole
Substituent Effects