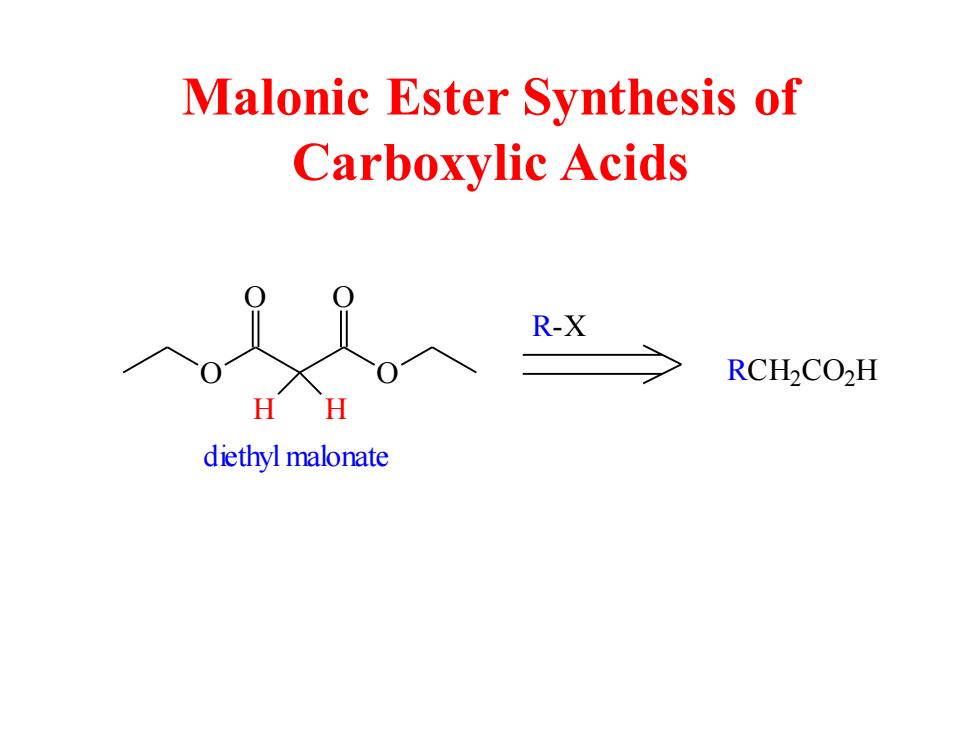
Malonic Ester Synthesis of Carboxylic Acids R-X RCH2CO>H H H diethyl malonate
Malonic Ester Synthesis of Carboxylic Acids O O O O H H diethyl malonate R- X RC H2 C O2H
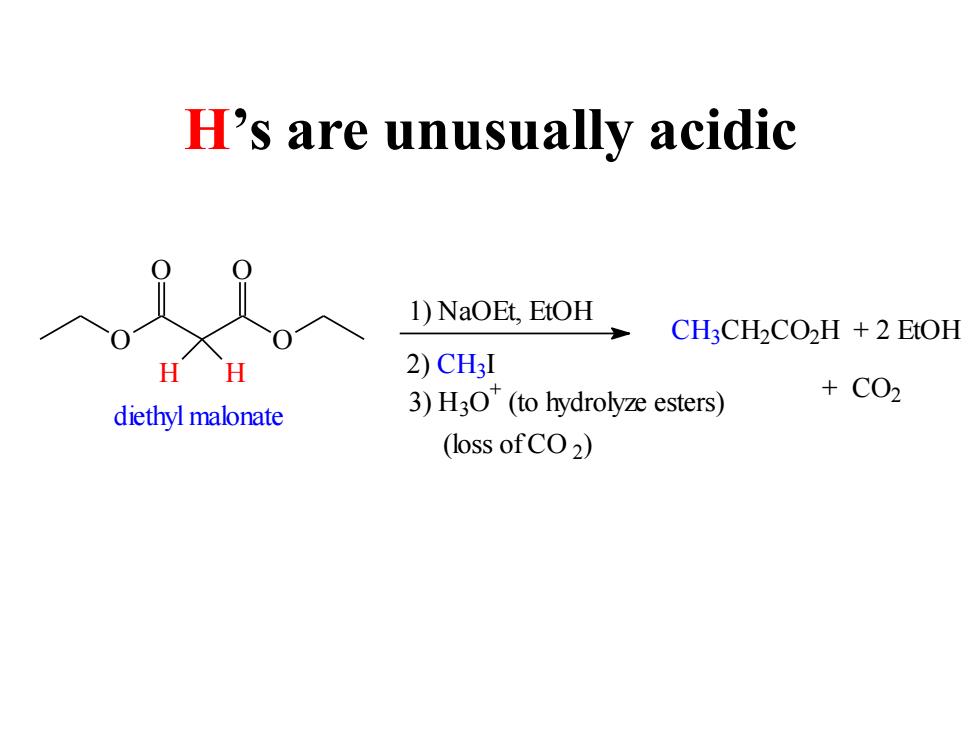
H's are unusually acidic 1)NaOEt,EtOH CH:CH2CO2H +2 EtOH H 2)CH3I +C02 diethyl malonate 3)H3O(to hydrolyze esters) (loss ofCO2)
H’s are unusually acidic O O O O H H diethyl malonate 1) NaOEt, EtOH 2) C H3I 3) H3O + (to hydrolyze esters) (loss of CO 2 ) C H3C H2C O2H + 2 EtOH + CO2
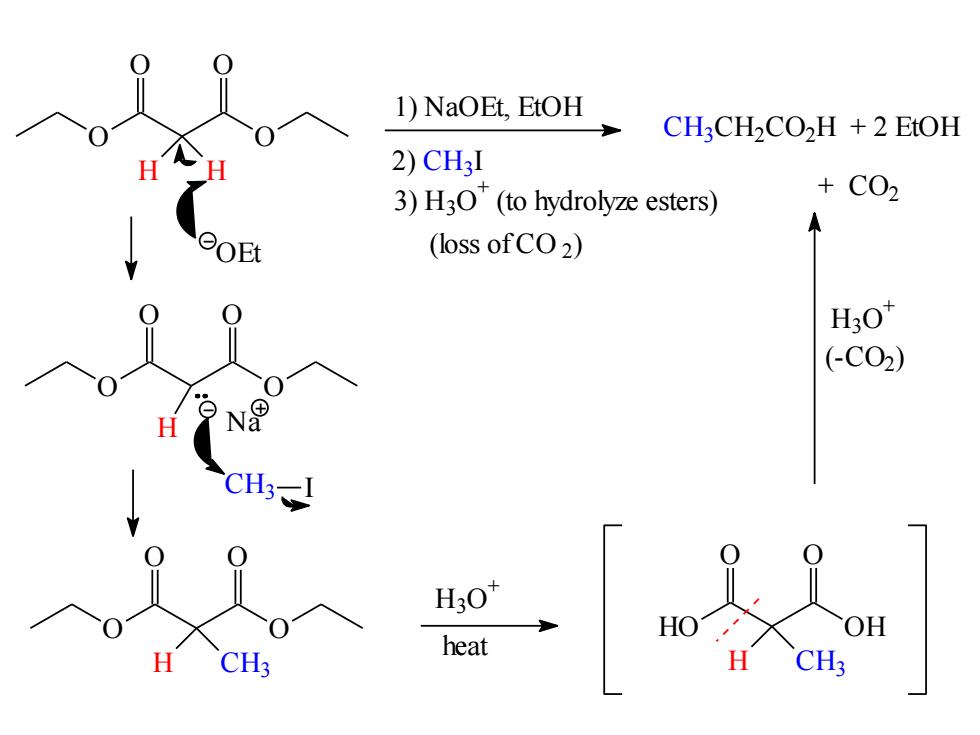
1)NaOEt,EtOH CH3CH2CO2H +2 EtOH 2)CH3I 3)H3O(to hydrolyze esters) +C02 (loss ofCO2) H3O (-C02) H3-1 CH heat
O O O O H H 1) NaOEt, EtOH 2) C H3 I 3) H3O + (to hydrolyze esters) (loss of CO 2) C H3C H2C O2H + 2 EtOH + CO2 OEt O O O O H N a C H3 I O O O O H CH3 H3O + HO OH O O H CH3 heat H3O + (-CO2)
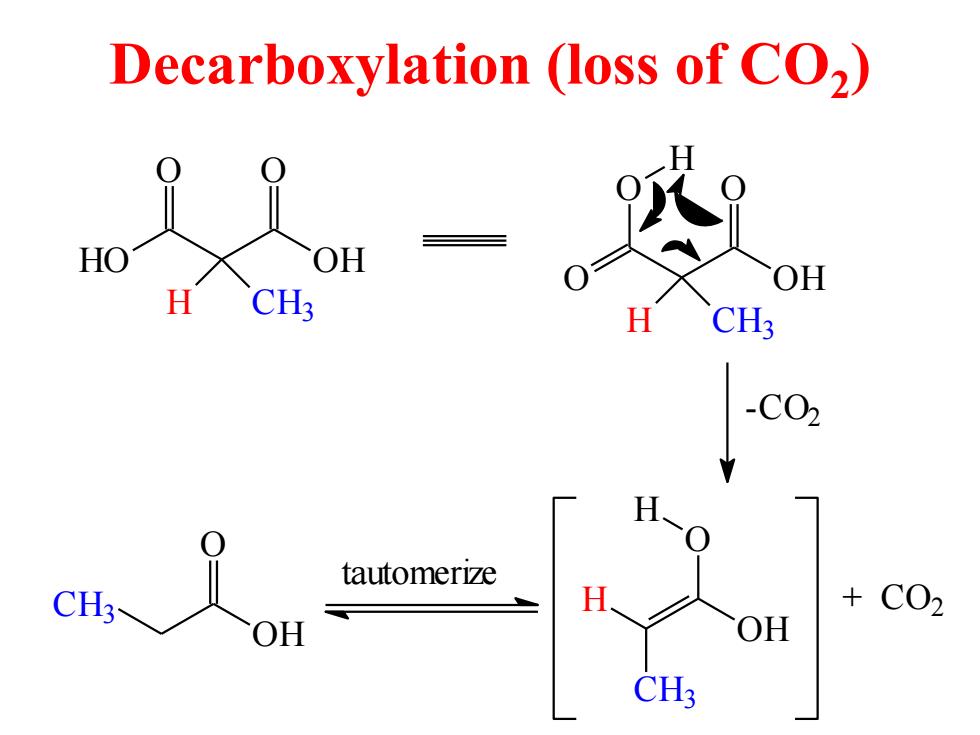
Decarboxylation (loss of CO2) HO OH H CH3- C02 OH OH CH?
Decarboxylation (loss of CO2 ) HO OH O O H CH3 O OH O O H CH3 H -CO2 O H OH CH3 H + CO2 tautomerize CH3 OH O