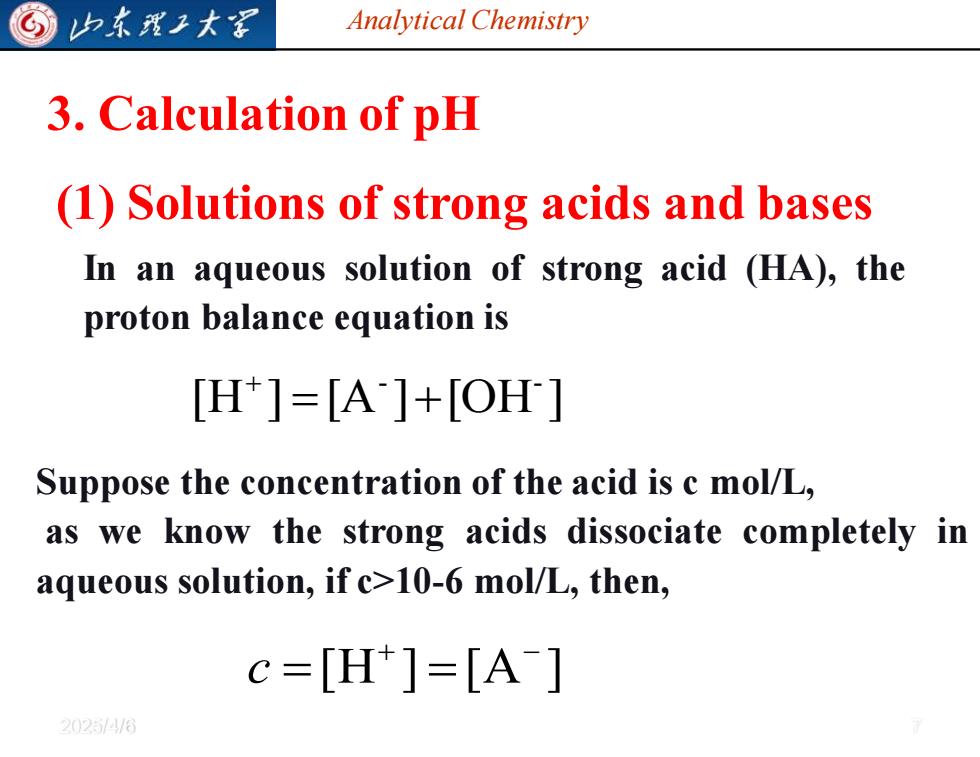
归东理工大军 Analytical Chemistry 3.Calculation of pH (1)Solutions of strong acids and bases In an aqueous solution of strong acid (HA),the proton balance equation is [H]=[A]+[OH] Suppose the concentration of the acid is c mol/L, as we know the strong acids dissociate completely in aqueous solution,if c>10-6 mol/L,then, c=[H]=[A] 20254/6
Analytical Chemistry 2025/4/6 7 3. Calculation of pH (1) Solutions of strong acids and bases In an aqueous solution of strong acid (HA), the proton balance equation is [H ] [A ] [OH ] - - = + + Suppose the concentration of the acid is c mol/L, as we know the strong acids dissociate completely in aqueous solution, if c>10-6 mol/L, then, [H ] [A ] + − c = =

G归东理工大军 Analytical Chemistry if c<10-6 mol/L, The autoprotolysis of water should not be neglected, [H]=c+ Kv [H] []=C++4K 2 For strong bases,similar equations can be derived for calculation of [OH-]. 20254/0
Analytical Chemistry 2025/4/6 8 if c<10-6 mol/L, The autoprotolysis of water should not be neglected, [H ] [H ] + + = + Kw c 2 4 [H ] 2 Kw c + c + = + For strong bases, similar equations can be derived for calculation of [OH- ]

归东理工大买 Analytical Chemistry (2)Solutions of weak acids and bases The pH of a weak acid can be calculated from the dissociation constant,K Similarly,the pH of a weak base can be calculated from its K value. For a strong bases,similar equations can be derived for calculation of [OH-]. For a weak acid,HA,we can write its proton balance equation: [H]=[A]+[OH] 20254/6
Analytical Chemistry 2025/4/6 9 (2) Solutions of weak acids and bases The pH of a weak acid can be calculated from the dissociation constant, Ka . Similarly, the pH of a weak base can be calculated from its Kb value. For a strong bases, similar equations can be derived for calculation of [OH- ]. For a weak acid, HA, we can write its proton balance equation: [H ] [A ] [OH ] - - = + +
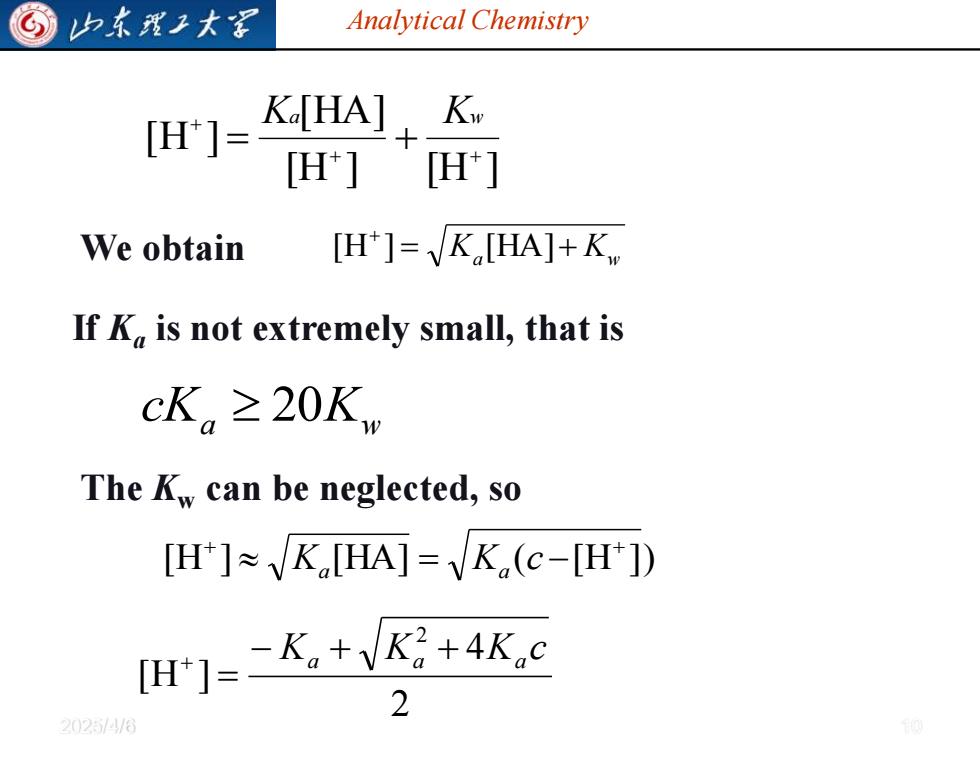
G 归东理工大军 Analytical Chemistry [H]= Ko[HA],K H'] H'] We obtain [H']=K[HA]+K If K is not extremely small,that is cK。≥20Kw The Kw can be neglected,so [H]K[HA]=K(c-[H]) H门=-K,+VK+4Kc 2 20254/0
Analytical Chemistry 2025/4/6 10 We obtain = Ka + Kw + [H ] [HA] a Kw cK 20[H ] [H ] [HA] [H ] + + + = + Ka Kw If Ka is not extremely small, that is The Kw can be neglected, so [H ] [HA] ( [H ]) + + Ka = Ka c − 2 4 [H ] 2 K K K c − a + a + a = +

山东理大 Analytical Chemistry Ifc/Ka≥400,the concentration of Ha can be considered c,the analytical concentration of the weak acid,then [H']=K.c This is the simplified equation. Similarly,the simplified equation of a weak base concentration is [OH ]=Kc 20254/6
Analytical Chemistry 2025/4/6 11 If c/Ka ≥400, the concentration of HA can be considered c, the analytical concentration of the weak acid, then K c = a + [H ] This is the simplified equation. Similarly, the simplified equation of a weak base concentration is K c = b − [OH ]