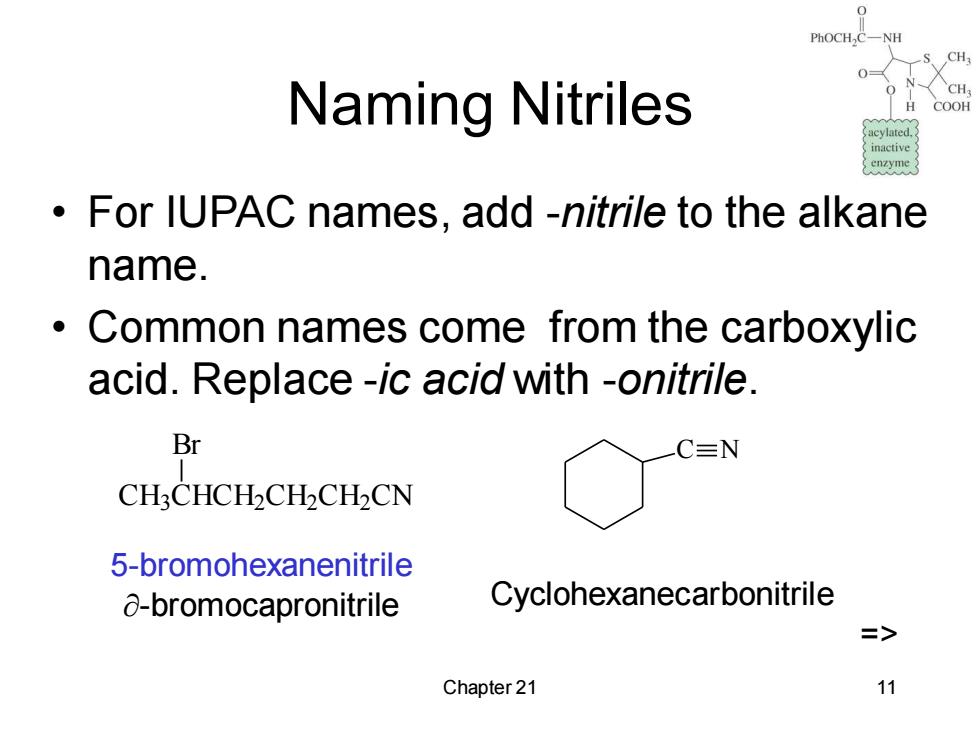
PhOCH,C一 Naming Nitriles COOH inactive enzyme For IUPAC names,add -nitrile to the alkane name. Common names come from the carboxylic acid.Replace -ic acid with -onitrile. Br C≡N CH:CHCH2CH2CH2CN 5-bromohexanenitrile 8-bromocapronitrile Cyclohexanecarbonitrile => Chapter 21 11
Chapter 21 11 Naming Nitriles • For IUPAC names, add -nitrile to the alkane name. • Common names come from the carboxylic acid. Replace -ic acid with -onitrile. CH3 CHCH2 CH2 CH2 CN Br 5-bromohexanenitrile -bromocapronitrile C N Cyclohexanecarbonitrile =>

0 PhOCH,C- NH CH CH COOH Acid Halides acylated. inactive enzyme More reactive than acids;the halogen withdraws e density from carbonyl. Named by replacing -ic acid with -yl halide. Br CI CH3CHCH2C-Br 3-bromobutanoyl bromide benzoyl chloride B-bromobutyryl bromide => Chapter 21 12
Chapter 21 12 Acid Halides • More reactive than acids; the halogen withdraws e- density from carbonyl. • Named by replacing -ic acid with -yl halide. C O Cl CH3 CHCH2 C Br O Br benzoyl chloride 3-bromobutanoyl bromide -bromobutyryl bromide =>
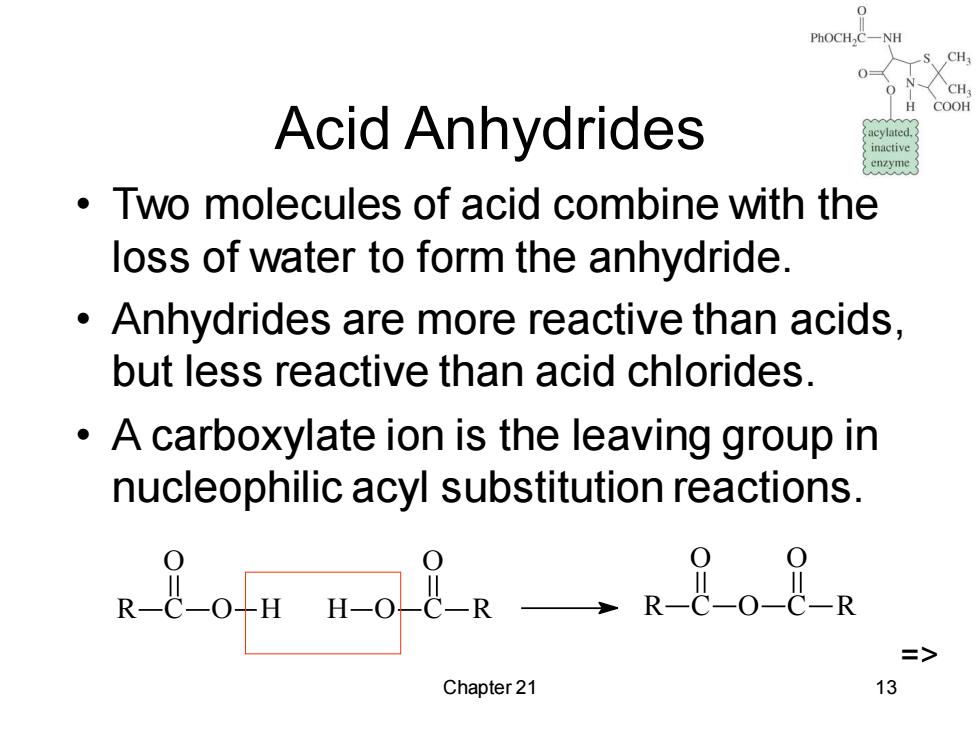
COOH Acid Anhydrides inactive enzyme Two molecules of acid combine with the loss of water to form the anhydride. Anhydrides are more reactive than acids. but less reactive than acid chlorides. A carboxylate ion is the leaving group in nucleophilic acyl substitution reactions. -2mo8g一·e8o8a => Chapter 21 13
Chapter 21 13 Acid Anhydrides • Two molecules of acid combine with the loss of water to form the anhydride. • Anhydrides are more reactive than acids, but less reactive than acid chlorides. • A carboxylate ion is the leaving group in nucleophilic acyl substitution reactions. R C O O H C R O H O R C O O C O R =>

0 PhOCH C CH CH COOH Naming Anhydrides mnactive enzyme The word acid is replaced with anhydride. For a mixed anhydride,name both acids. Diacids may form anhydrides if a 5-or 6- membered ring is the product. enr 2 o 2cm ethanoic anhydride acetic anhydride 1,2-benzenedicarboxylic anhydride phthalic anhydride => Chapter 21 14
Chapter 21 14 Naming Anhydrides • The word acid is replaced with anhydride. • For a mixed anhydride, name both acids. • Diacids may form anhydrides if a 5- or 6- membered ring is the product. CH3 C O O C O CH3 ethanoic anhydride acetic anhydride O O O 1,2-benzenedicarboxylic anhydride phthalic anhydride =>
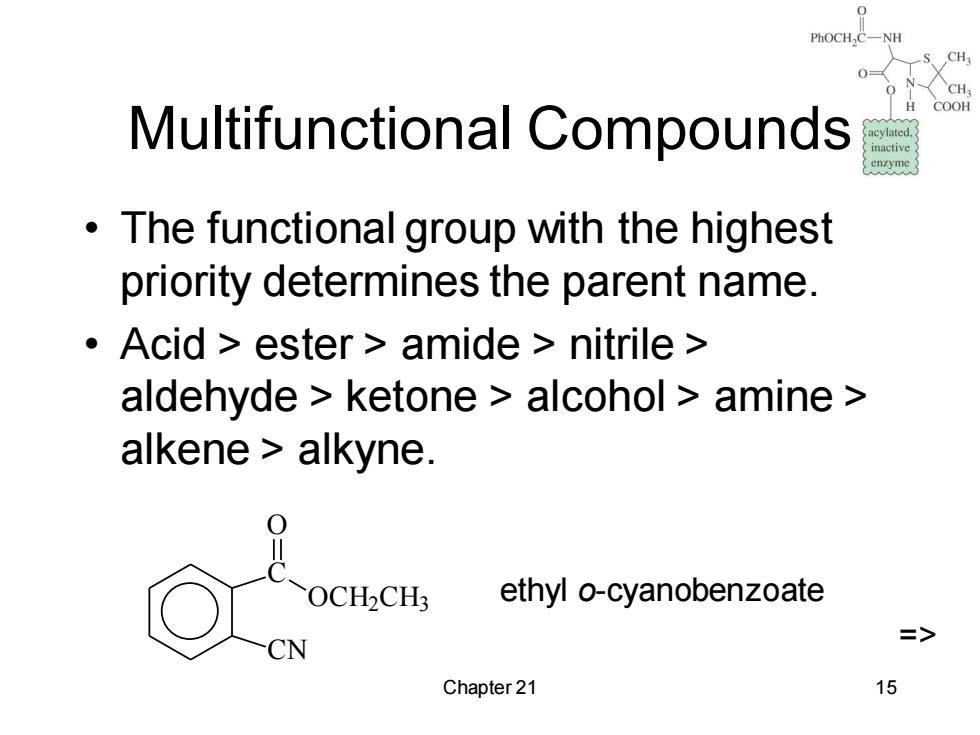
PhOCH,C-NH 0 H COOH Multifunctional Compounds acylated. inactive enzyme The functional group with the highest priority determines the parent name. Acid ester amide nitrile aldehyde ketone alcohol amine alkene alkyne. ! OCH2CH: ethyl o-cyanobenzoate => CN Chapter 21 15
Chapter 21 15 Multifunctional Compounds • The functional group with the highest priority determines the parent name. • Acid > ester > amide > nitrile > aldehyde > ketone > alcohol > amine > alkene > alkyne. C CN O OCH2CH3 ethyl o-cyanobenzoate =>