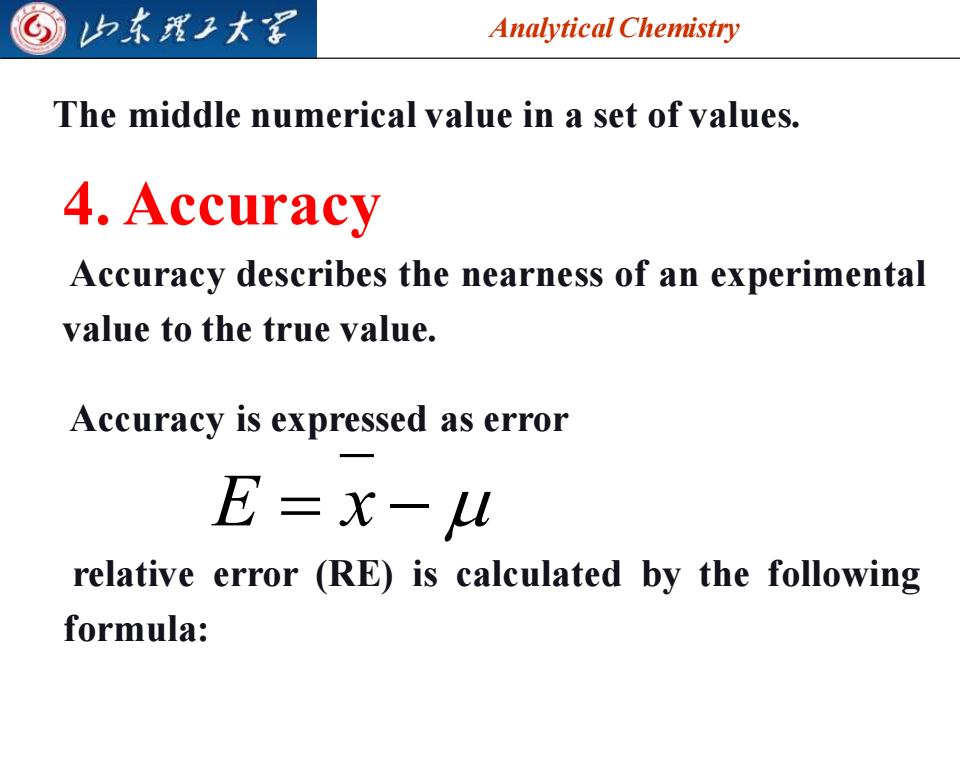
山东理子大军 Analytical Chemistry The middle numerical value in a set of values. 4.Accuracy Accuracy describes the nearness of an experimental value to the true value. Accuracy is expressed as error E=x- relative error (RE)is calculated by the following formula:
Analytical Chemistry 2025/4/3 6 4. Accuracy E = x − Accuracy is expressed as error relative error (RE) is calculated by the following formula: The middle numerical value in a set of values. Accuracy describes the nearness of an experimental value to the true value
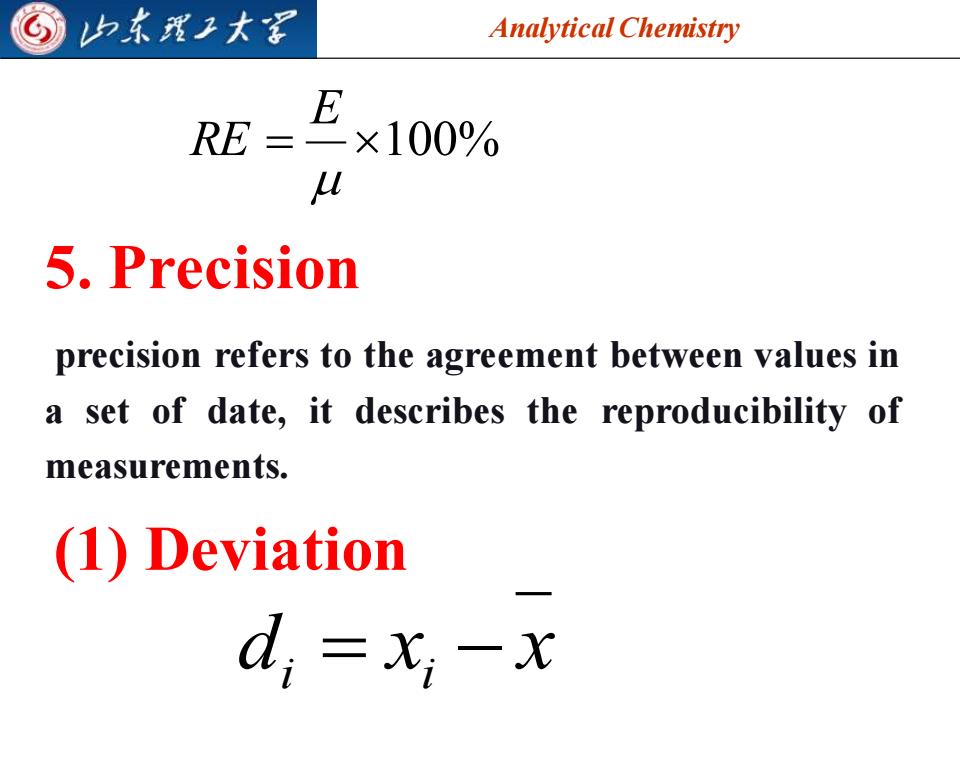
G 归东理子大图 Analytical Chemistry E RE=×100% U 5,Precision precision refers to the agreement between values in a set of date,it describes the reproducibility of measurements. (1)Deviation d;=xi-x
Analytical Chemistry 2025/4/3 7 = 100% E RE 5. Precision precision refers to the agreement between values in a set of date, it describes the reproducibility of measurements. (1) Deviation d x x i = i −

归东理王大军 Analytical Chemistry (2)Relative deviation RD=4×100% X (③)average deviation Σx-刘 d= n
Analytical Chemistry 2025/4/3 8 (2) Relative deviation = 100% x d RD i (3) average deviation n x x d n i i = − = 1
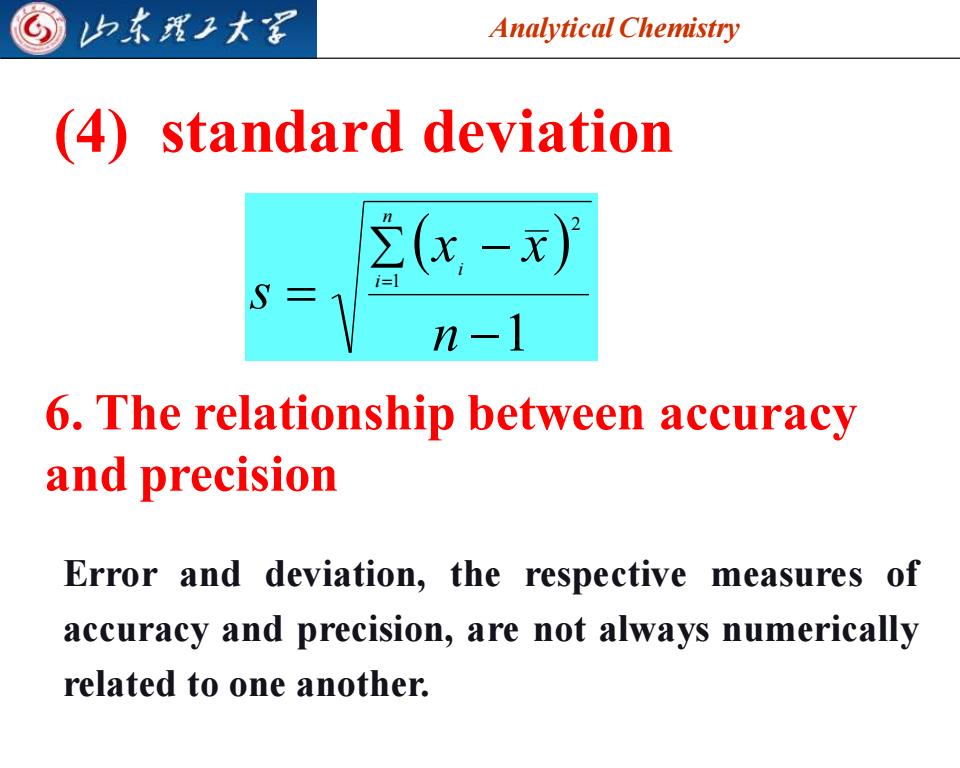
归东理子大军 Analytical Chemistry (4)standard deviation n-1 6.The relationship between accuracy and precision Error and deviation,the respective measures of accuracy and precision,are not always numerically related to one another
Analytical Chemistry 2025/4/3 9 (4) standard deviation ( ) 1 1 2 − − = = n x x s n i i 6. The relationship between accuracy and precision Error and deviation, the respective measures of accuracy and precision, are not always numerically related to one another

山东理工大军 Analytical Chemistry That is,the precision of the data in a set can be excellent while the overall accuracy is terrible. The precision must be good if we want to get a high accuracy
Analytical Chemistry 2025/4/3 10 That is, the precision of the data in a set can be excellent while the overall accuracy is terrible. The precision must be good if we want to get a high accuracy