
4πelectrons 6πelectrons 8πelectrons not aromatic aromatic not aromatic 6πelectrons Planar... aromatic 6πelectrons
College of Science
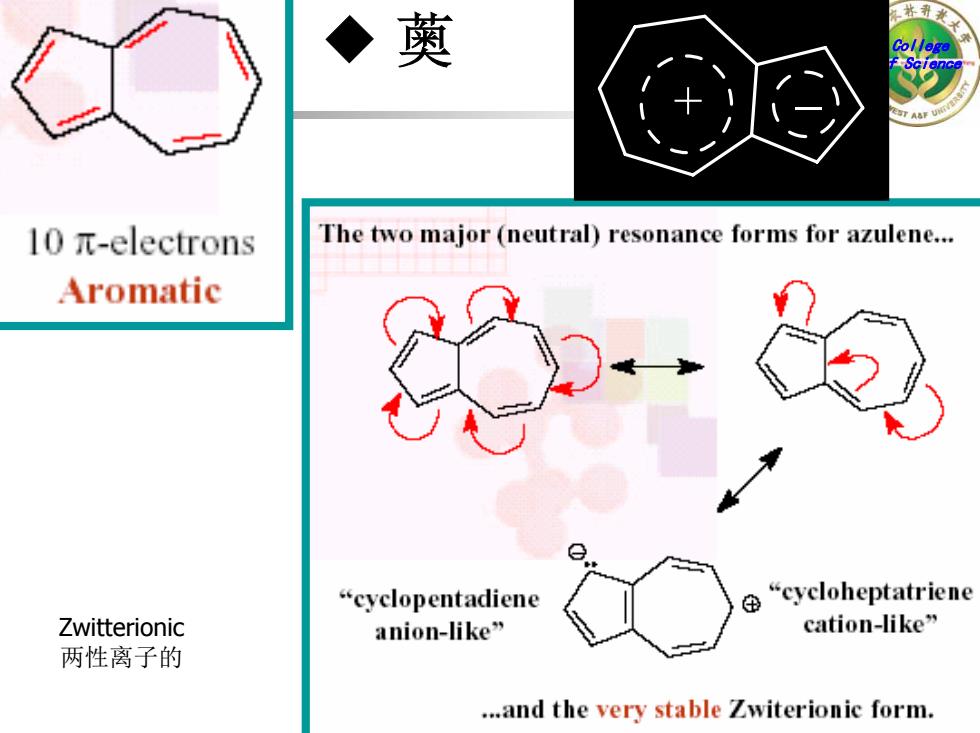
薁 l0π-electrons The two major (neutral)resonance forms for azulene... Aromatic “cyclopentadiene “cycloheptatriene Zwitterionic anion-ike” cation-ike” 两性离子的 ...and the very stable Zwiterionic form
College of Science + _ ◆ 薁 Zwitterionic 两性离子的
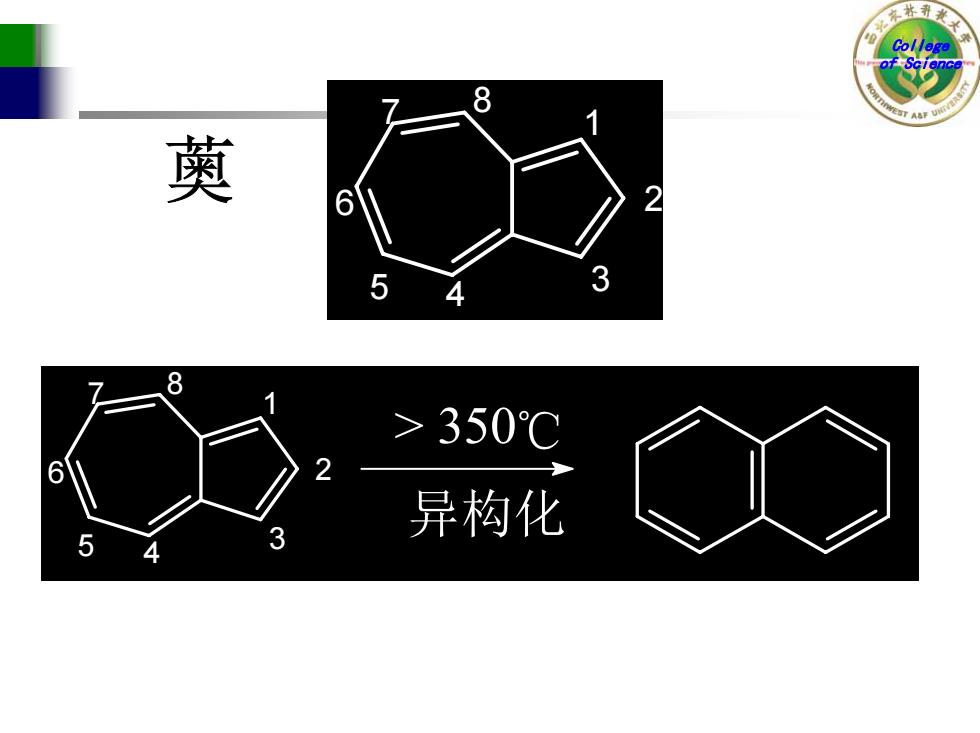
8 萸 5 3 8 >350℃ 2 异构化 5 3 4
College of Science 1 2 3 5 4 6 7 8 薁 1 2 3 5 4 6 7 8 > 350 ℃ 异构 化
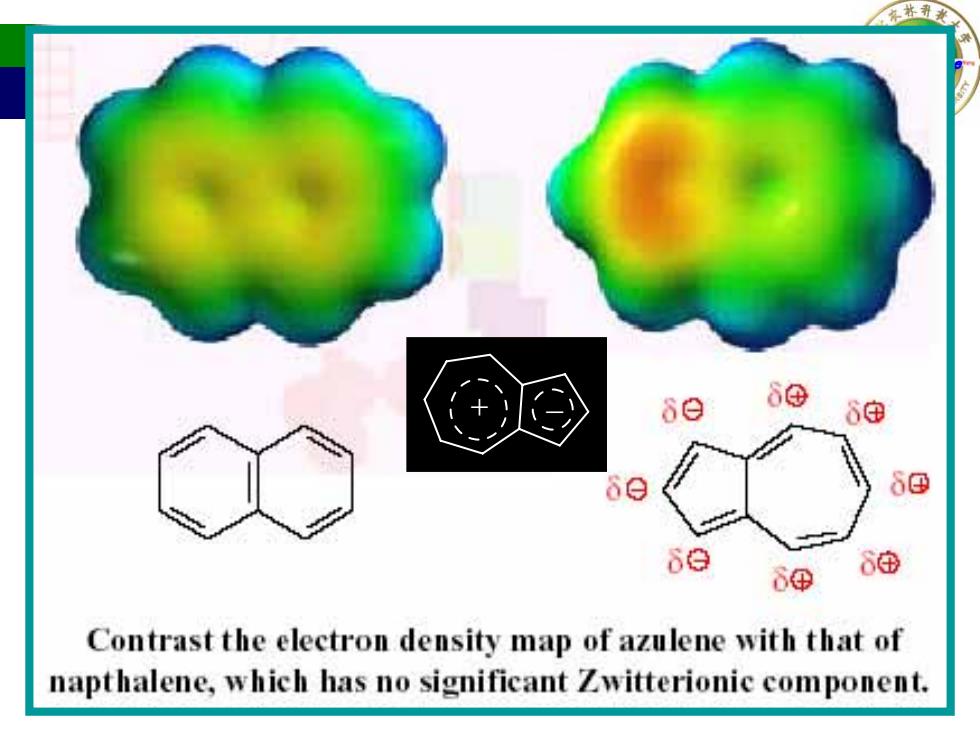
80 se 8e 6④ 6由 Contrast the electron density map of azulene with that of napthalene,which has no significant Zwitterionic component
College of Science + _
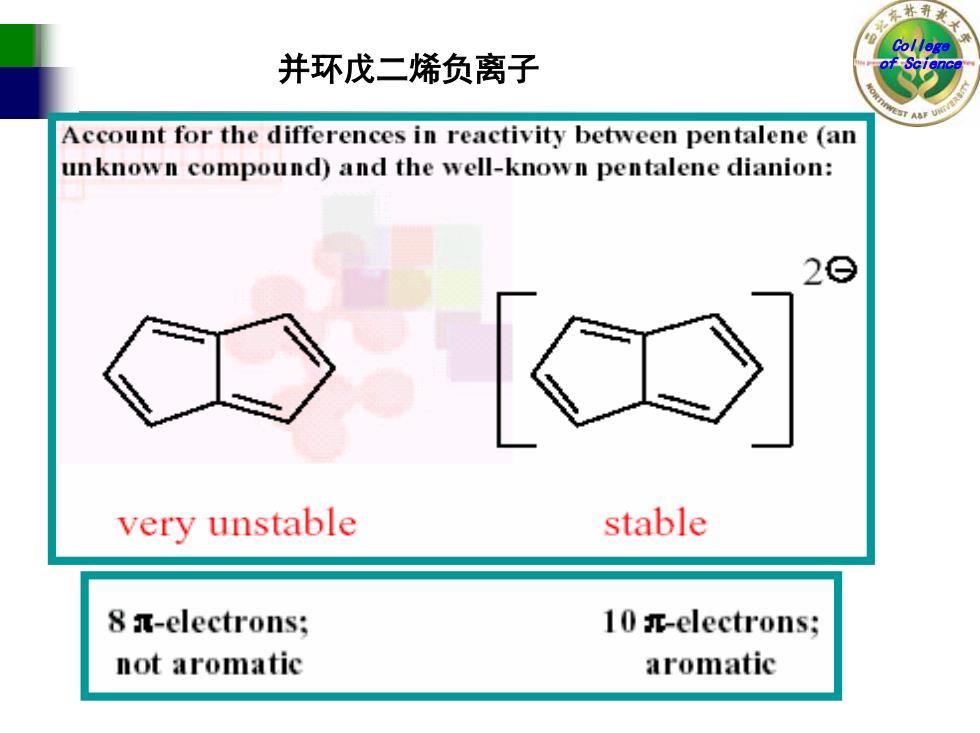
东林 College 并环戊二烯负离子 Account for the differences in reactivity between pentalene (an unknown compound)and the well-known pentalene dianion: 29 k very unstable stable 8 a-electrons; 10 -electrons; not aromatic aromatic
College 并环戊二烯负离子 of Science