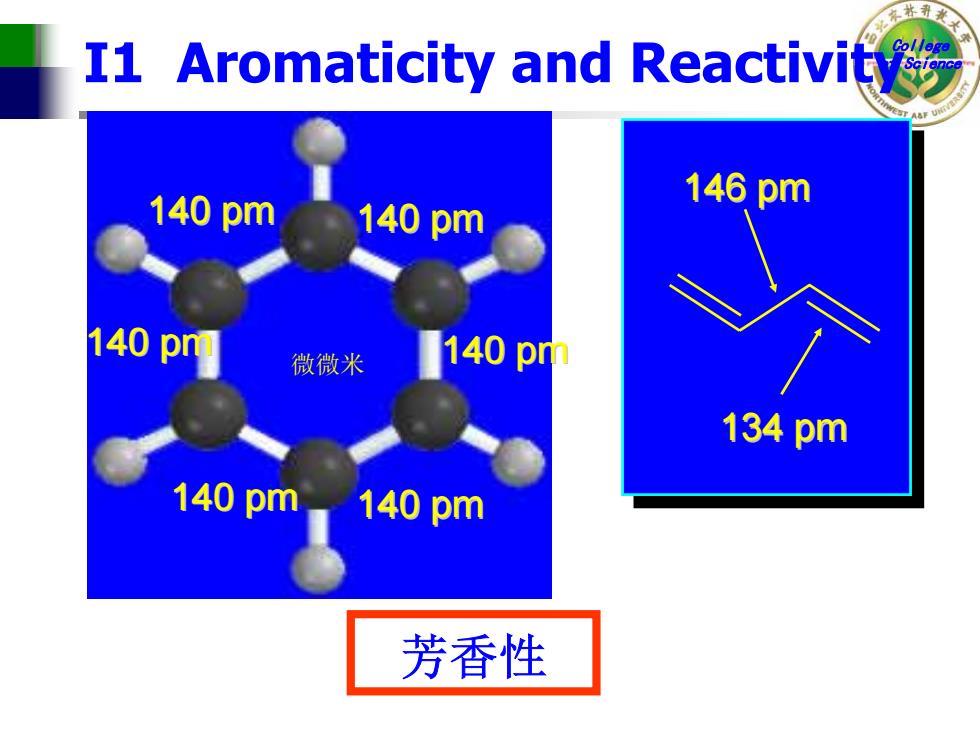
I1 Aromaticity and Reactivity 140pm 146pm 140pm 140pm 微微米 140pm 134pm 140pm140pm 芳香性
College of Science I1 Aromaticity and Reactivity 140 pm 140 pm 140 pm 140 pm 140 pm 140 pm 146 pm 134 pm 微微米 芳香性
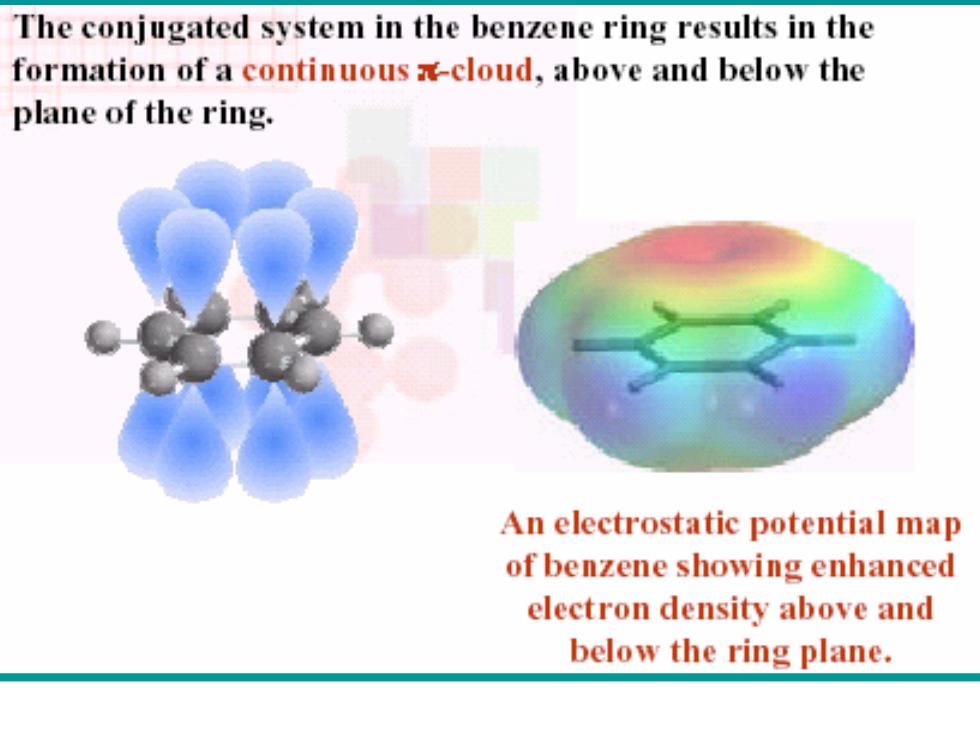
The conjugated system in the benzene ring results in the formation of a continuous -cloud,above and below the plane of the ring. An electrostatic potential map of benzene showing enhanced electron density above and below the ring plane
College of Science
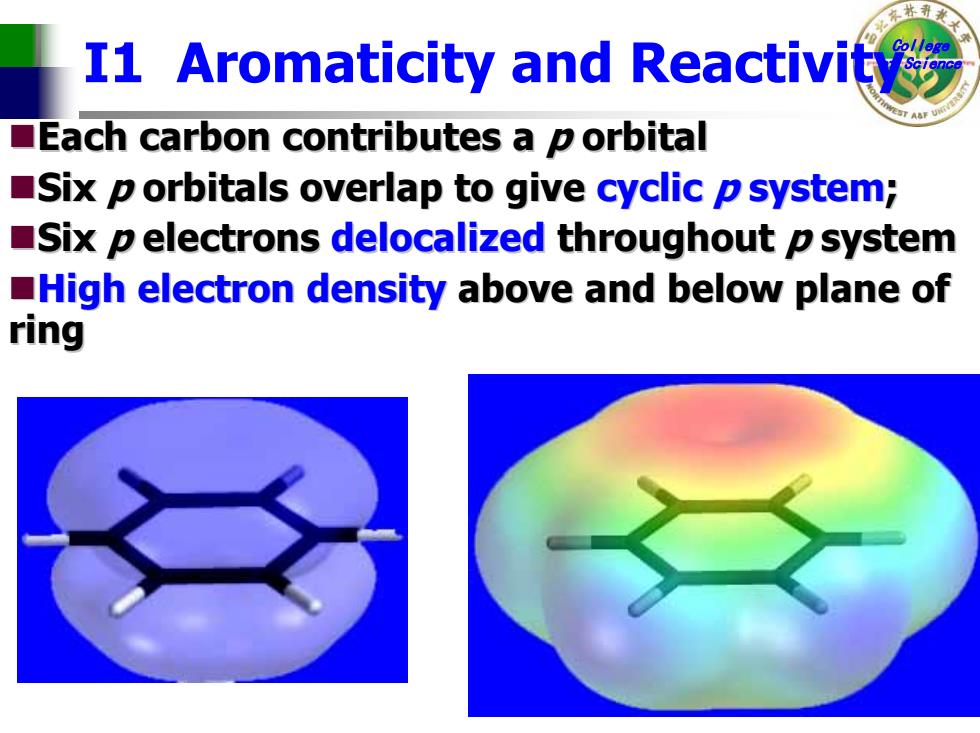
I1 Aromaticity and Reactivity Each carbon contributes a p orbital Six p orbitals overlap to give cyclic p system; Six p electrons delocalized throughout p system High electron density above and below plane of ring
College of Science I1 Aromaticity and Reactivity Each carbon contributes a Each carbon contributes a p orbital orbital Six p orbitals orbitals overlap to give overlap to give cyclic p system ; Six p electrons electrons delocalized delocalized throughout throughout p system High electron density High electron density above and below plane of above and below plane of ring

I1 Aromaticity and Reactivity ■2.反应性 ●不易加成(苯环骨架不断开) ●发生取代(亲电取代E.S) ●侧链反应 ■3.芳香性化合物结构特征 >大的不饱和度(2≥4)2=? >(环状)平面分子 >易进行亲电取代反应 芳香性
College of Science I1 Aromaticity and Reactivity 2. 反应性 z不易加成 (苯环骨架不断开 ) z发生取代 (亲电取代 E.S) z侧链反应 3. 芳香性化合物结构特征 ¾大的不饱和度 ( Ω ≧4) Ω=? ¾ (环状 )平面分子 ¾易进行亲电取代反应 芳香性
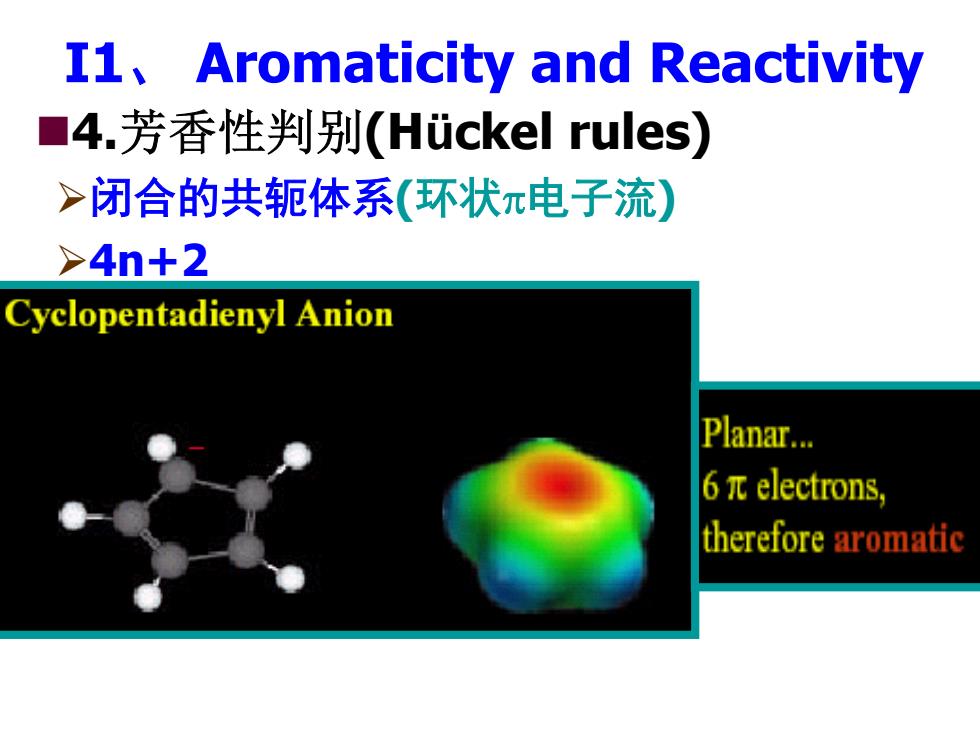
I1,Aromaticity and Reactivity ■4.芳香性判别(Huckel rules) >闭合的共轭体系(环状π电子流) >4n+2 Cyclopentadienyl Anion Planar... 6πelectrons, therefore aromatic
I1、 Aromaticity and Reactivity 4.芳香性判别(Hückel rules) ¾闭合的共轭体系(环状π电子流) ¾4n+2