
Sec 1 Preparation of RX Free radical halogenation produces mixtures,not good lab synthesis ounless:all H's are equivalent,or ohalogenation is highly selective. Free radical allylic halogenation oproduces alkyl halide with double bond on the neighboring carbon
Sec 1 Preparation of RX ◼Free radical halogenation ⚫produces mixtures, not good lab synthesis ⚫unless: all H’s are equivalent, or ⚫halogenation is highly selective. ◼Free radical allylic halogenation ⚫produces alkyl halide with double bond on the neighboring carbon
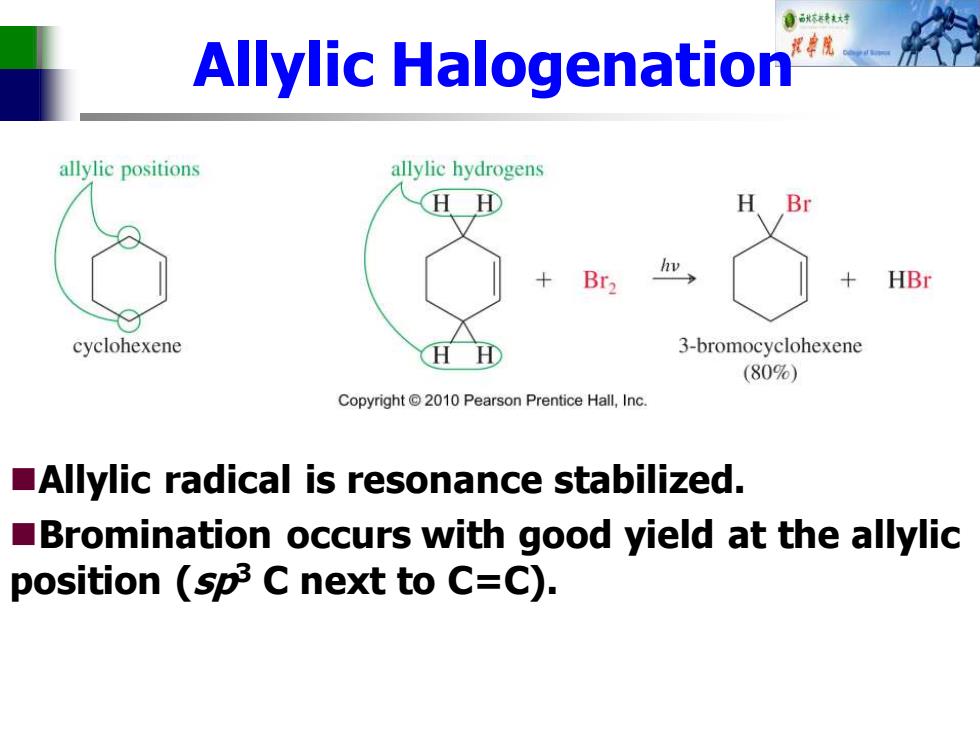
自秋转达对 Allylic Halogenation allylic positions allylic hydrogens HH H Br2 w HBr cyclohexene H 3-bromocyclohexene (80%) Copyright 2010 Pearson Prentice Hall,Inc. Allylic radical is resonance stabilized. Bromination occurs with good yield at the allylic position (sp3 C next to C=C)
Allylic Halogenation ◼Allylic radical is resonance stabilized. ◼Bromination occurs with good yield at the allylic position (sp3 C next to C=C)
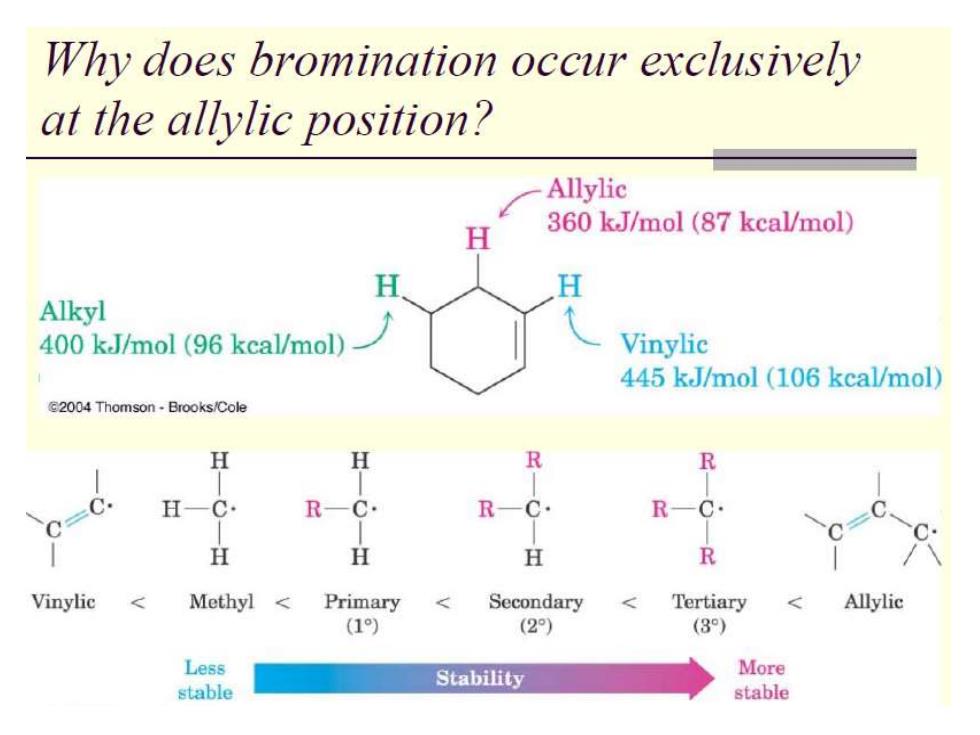
Why does bromination occur exclusively at the allylic position? Allylic H 360 kJ/mol (87 kcal/mol) H Alkyl 400 kJ/mol (96 kcal/mol) Vinylic 445 kJ/mol (106 kcal/mol) E2004 Thomson-Brooks/Cole H H R R H-C· R-C. R-C. R-C. H H H R Vinylic Methyl Primary Secondary < Tertiary Allylic (1) (2) (3) Less Stability More stable stable
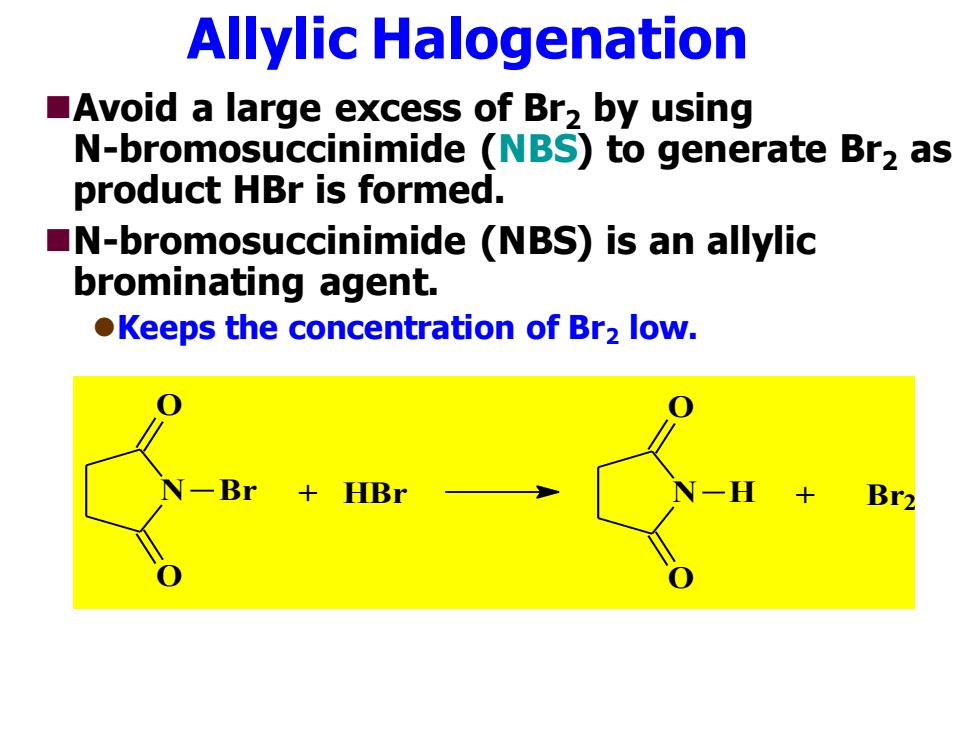
Allylic Halogenation Avoid a large excess of Br2 by using N-bromosuccinimide (NBS)to generate Br2 as product HBr is formed. N-bromosuccinimide (NBS)is an allylic brominating agent. Keeps the concentration of Br2 low. CBr+HBr Br2
Allylic Halogenation ◼Avoid a large excess of Br2 by using N-bromosuccinimide (NBS) to generate Br2 as product HBr is formed. ◼N-bromosuccinimide (NBS) is an allylic brominating agent. ⚫Keeps the concentration of Br2 low. N O O Br + HBr N O O H + Br2
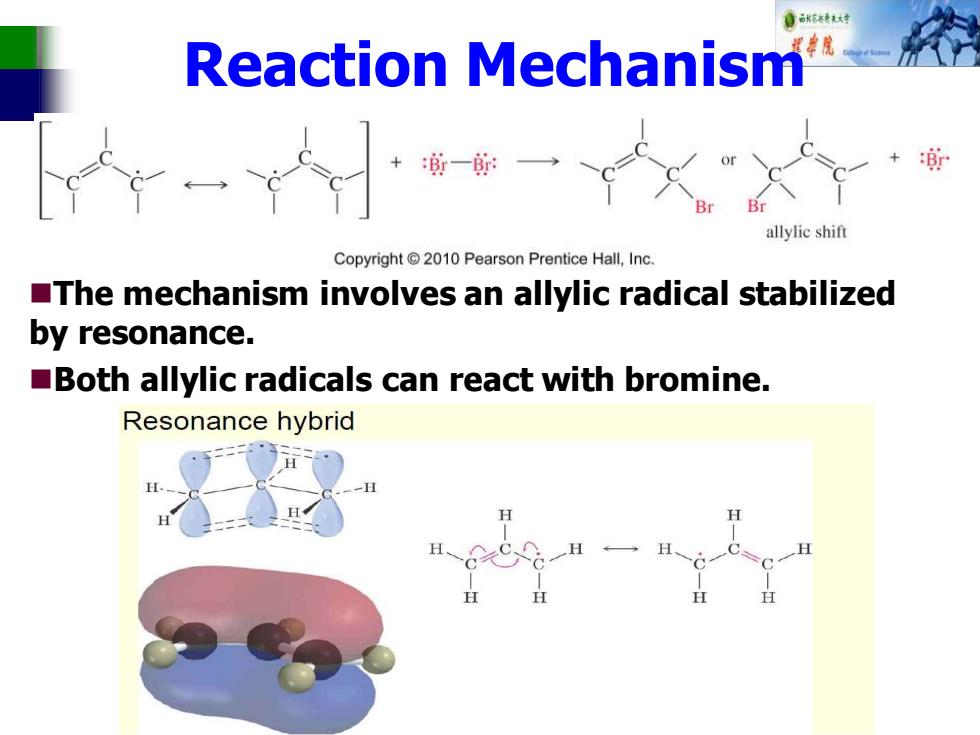
自秋特大材 Reaction Mechanism +一一 allylic shift Copyright2010 Pearson Prentice Hall,Inc. The mechanism involves an allylic radical stabilized by resonance. Both allylic radicals can react with bromine. Resonance hybrid
Reaction Mechanism ◼The mechanism involves an allylic radical stabilized by resonance. ◼Both allylic radicals can react with bromine