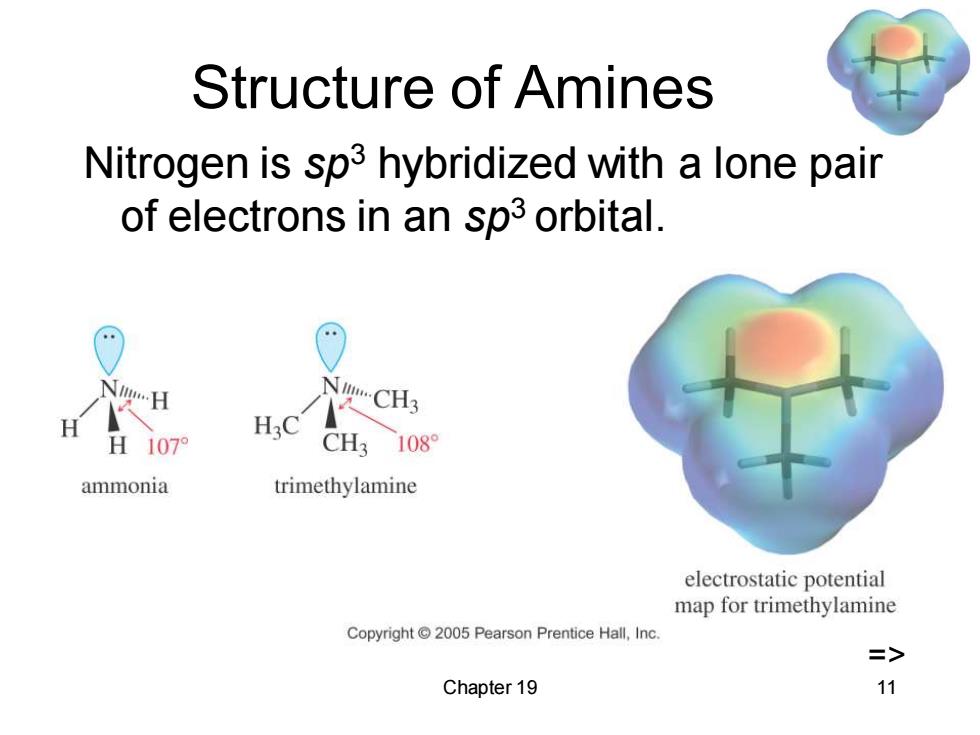
Structure of Amines Nitrogen is sp3 hybridized with a lone pair of electrons in an sp3 orbital. H3C 1079 CH3108 ammonia trimethylamine electrostatic potential map for trimethylamine Copyright 2005 Pearson Prentice Hall,Inc. => Chapter 19 11
Chapter 19 11 Structure of Amines Nitrogen is sp3 hybridized with a lone pair of electrons in an sp3 orbital. =>
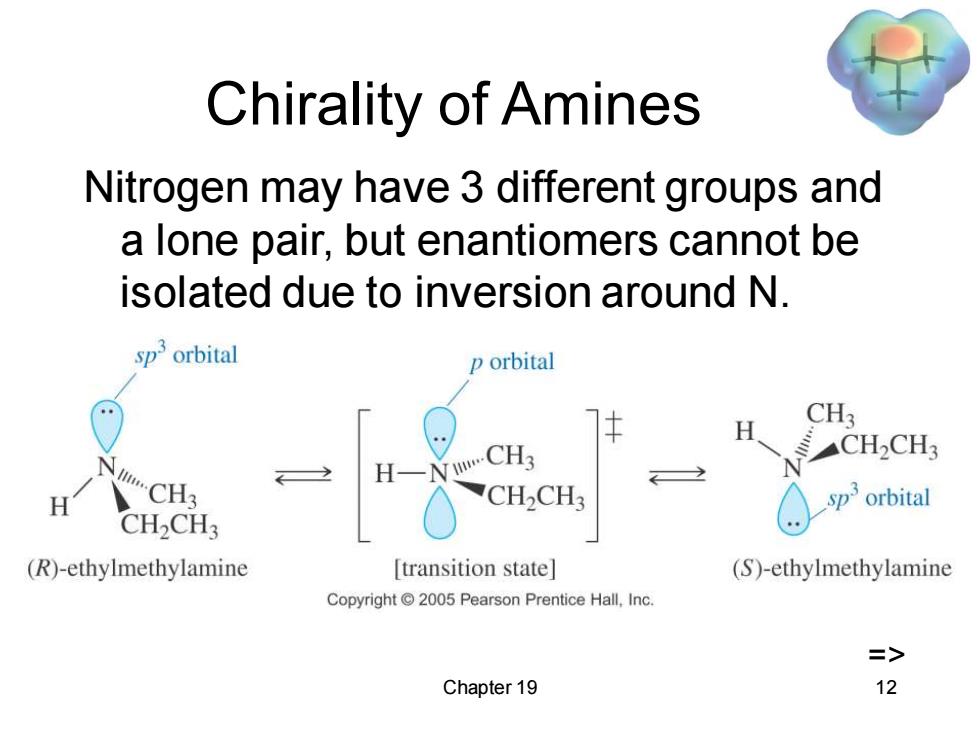
Chirality of Amines Nitrogen may have 3 different groups and a lone pair,but enantiomers cannot be isolated due to inversion around N. sporbital p orbital H CH3 CH2CH3 H wCH3 H CH2CH3 sporbital CH2CH3 (R)-ethylmethylamine [transition state] (S)-ethylmethylamine Copyright2005 Pearson Prentice Hall,Inc. => Chapter 19 12
Chapter 19 12 Chirality of Amines Nitrogen may have 3 different groups and a lone pair, but enantiomers cannot be isolated due to inversion around N. =>
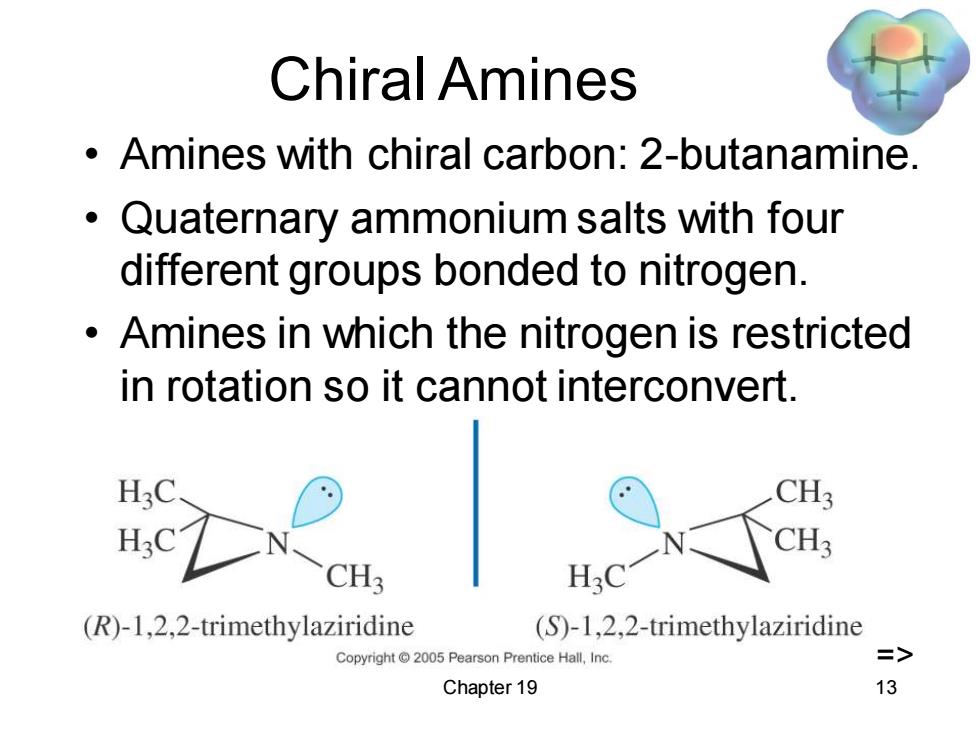
Chiral Amines Amines with chiral carbon:2-butanamine. Quaternary ammonium salts with four different groups bonded to nitrogen. Amines in which the nitrogen is restricted in rotation so it cannot interconvert. H3C CH3 H3C CH3 CH (R)-1,2,2-trimethylaziridine (S)-1,2,2-trimethylaziridine Copyright2005 Pearson Prentice Hall,Inc. => Chapter 19 13
Chapter 19 13 Chiral Amines • Amines with chiral carbon: 2-butanamine. • Quaternary ammonium salts with four different groups bonded to nitrogen. • Amines in which the nitrogen is restricted in rotation so it cannot interconvert. =>
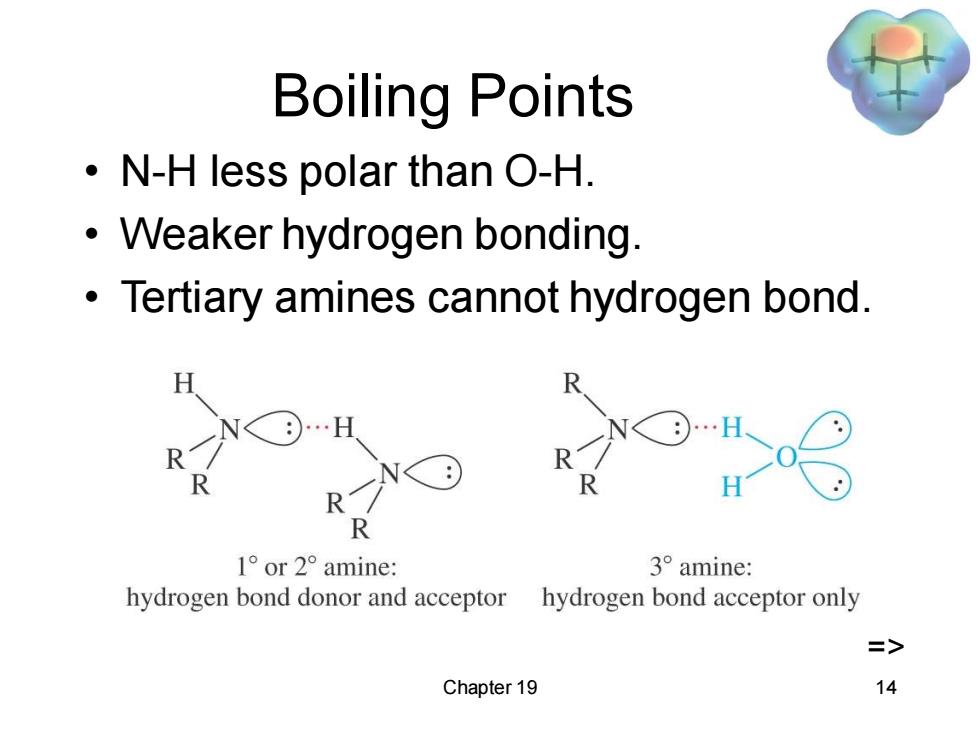
Boiling Points N-H less polar than O-H. Weaker hydrogen bonding. Tertiary amines cannot hydrogen bond. …H R R R 1°or2°amine: 3°amine: hydrogen bond donor and acceptor hydrogen bond acceptor only Chapter 19 14
Chapter 19 14 Boiling Points • N-H less polar than O-H. • Weaker hydrogen bonding. • Tertiary amines cannot hydrogen bond. =>

Solubility and Odor Small amines (<6 C)soluble in water. All amines accept hydrogen bonds from water and alcohol. Branching increases solubility. Most amines smell like rotting fish. NH2CH2CH2CH2CH2CH2NH2 1,5-pentanediamine or cadaverine => Chapter 19 15
Chapter 19 15 Solubility and Odor • Small amines (<6 C) soluble in water. • All amines accept hydrogen bonds from water and alcohol. • Branching increases solubility. • Most amines smell like rotting fish. NH2 CH2 CH2 CH2 CH2 CH2 NH2 1,5-pentanediamine or cadaverine =>