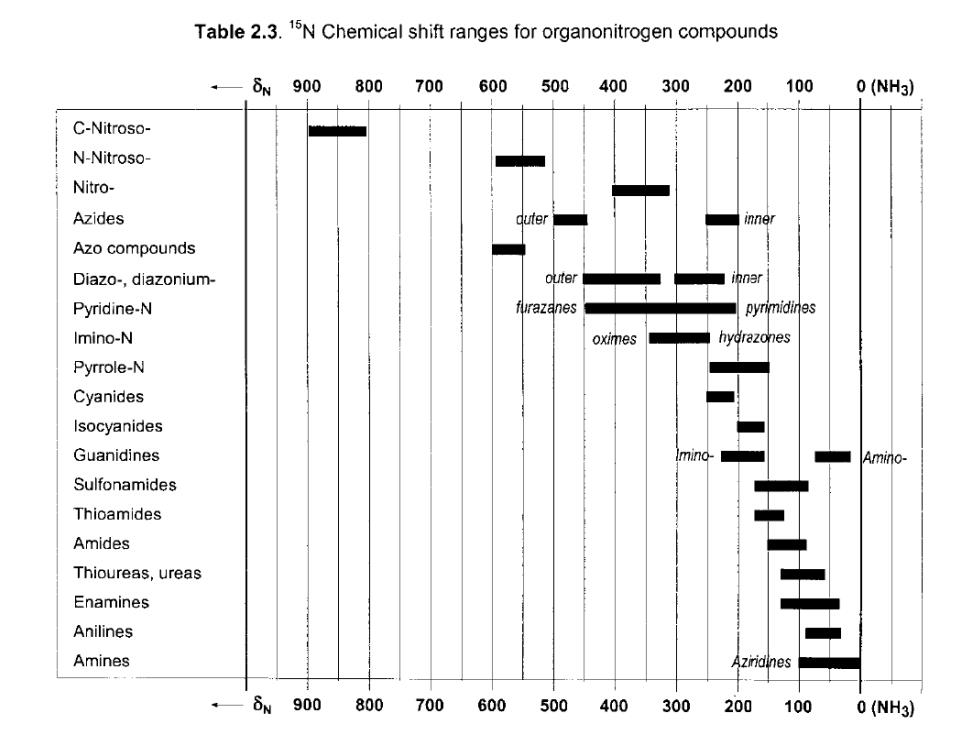
Table 2.3.15N Chemical shift ranges for organonitrogen compounds δN900 800700 600 500400 300 200 100 0(NH3) C-Nitroso- N-Nitroso- Nitro- Azides inner Azo compounds Diazo-,diazonium- ter Pyridine-N furazanes pyrimidines Imino-N oximes hydrazones Pyrrole-N Cyanides Isocyanides Guanidines Amino- Sulfonamides Thioamides Amides Thioureas,ureas Enamines Anilines Amines 8w900 800700600 500400300200100 0(NH3)
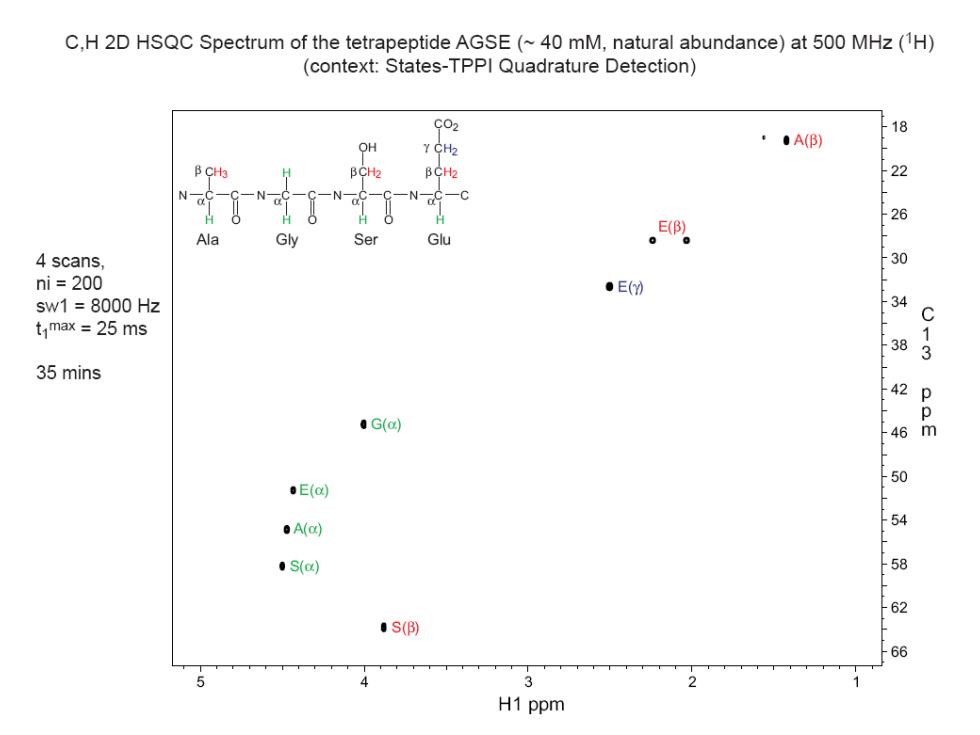
C,H 2D HSQC Spectrum of the tetrapeptide AGSE(~40 mM,natural abundance)at 500 MHz(1H) (context:States-TPPI Quadrature Detection) C02 ··A() ® OH Y CH2 B CH3 BCH2 BC比 22 N 一N 一● 26 Ala Gly Ser Glu 4 scans 30 ni=200 ●E() sw1=8000Hz 34 C t max =25 ms 38 35 mins 42 Q 0 ·G(c) 46m ·E(c ·A(c 54 ·S() 58 62 ·s() 66 4 3 H1 ppm
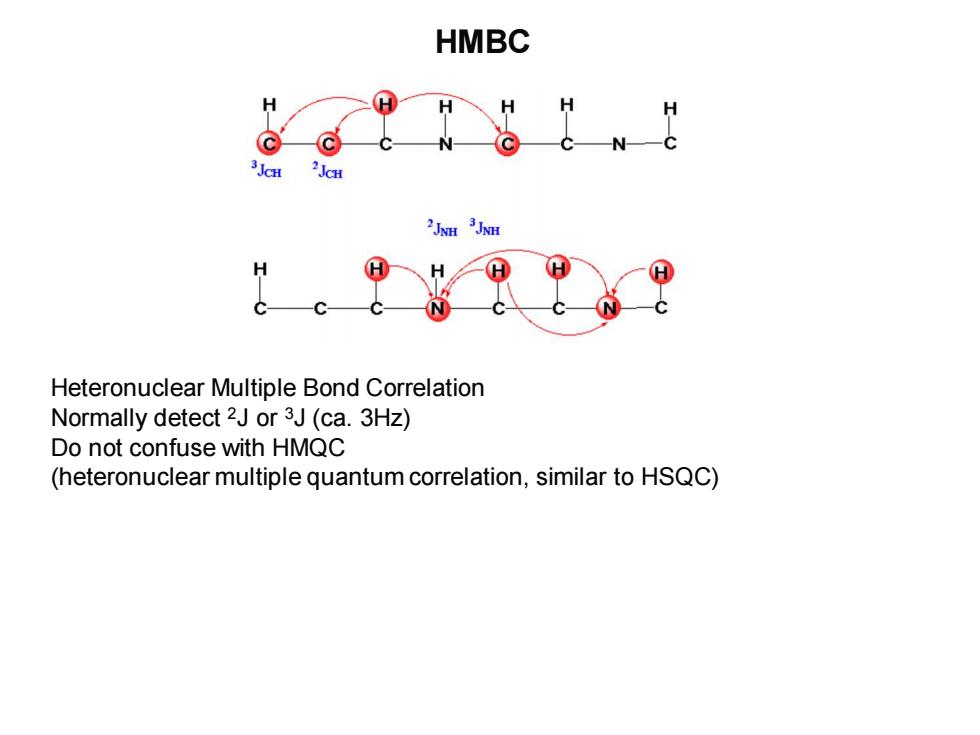
HMBC H H H℃ Heteronuclear Multiple Bond Correlation Normally detect 2J or 3J(ca.3Hz) Do not confuse with HMQC (heteronuclear multiple quantum correlation,similar to HSQC)
HMBC Heteronuclear Multiple Bond Correlation Normally detect 2J or 3J (ca. 3Hz) Do not confuse with HMQC (heteronuclear multiple quantum correlation, similar to HSQC)
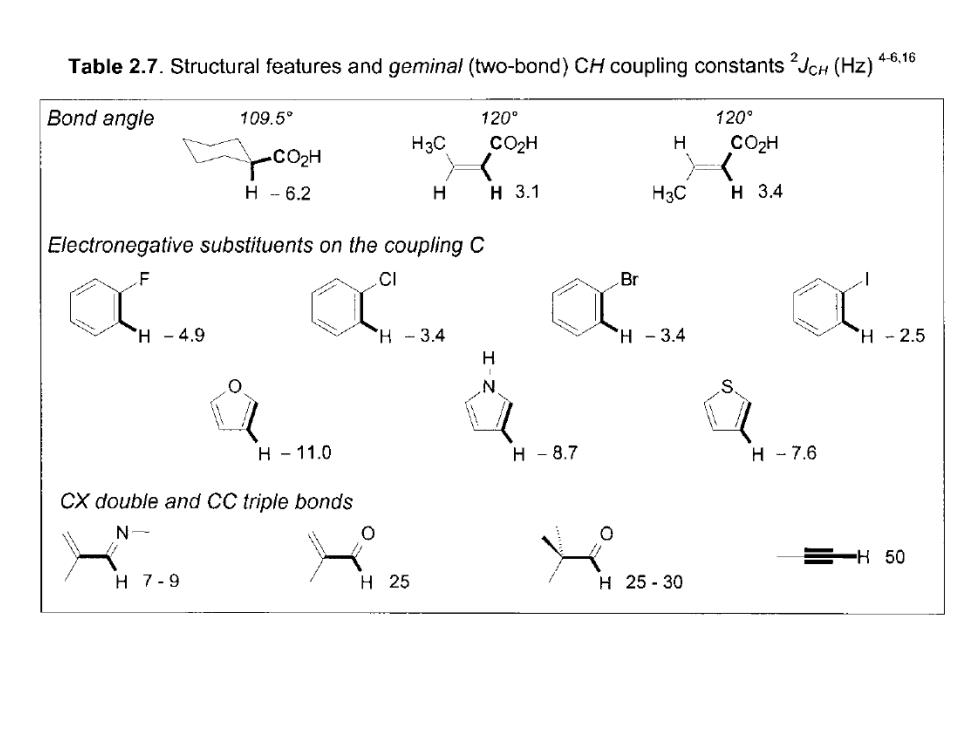
Table 2.7.Structural features and geminal(two-bond)CH coupling constants(Hz)4.16 Bond angle 109.5 120 120° CO2H H3C CO2H H CO2H H-6.2 H H3.1 H3C H3.4 Electronegative substituents on the coupling C CI Br H -4.9 -3.4 -3.4 2.5 H H-11.0 H -8.7 -7.6 CX double and CC triple bonds N 三-H50 H7-9 25 H 25.30
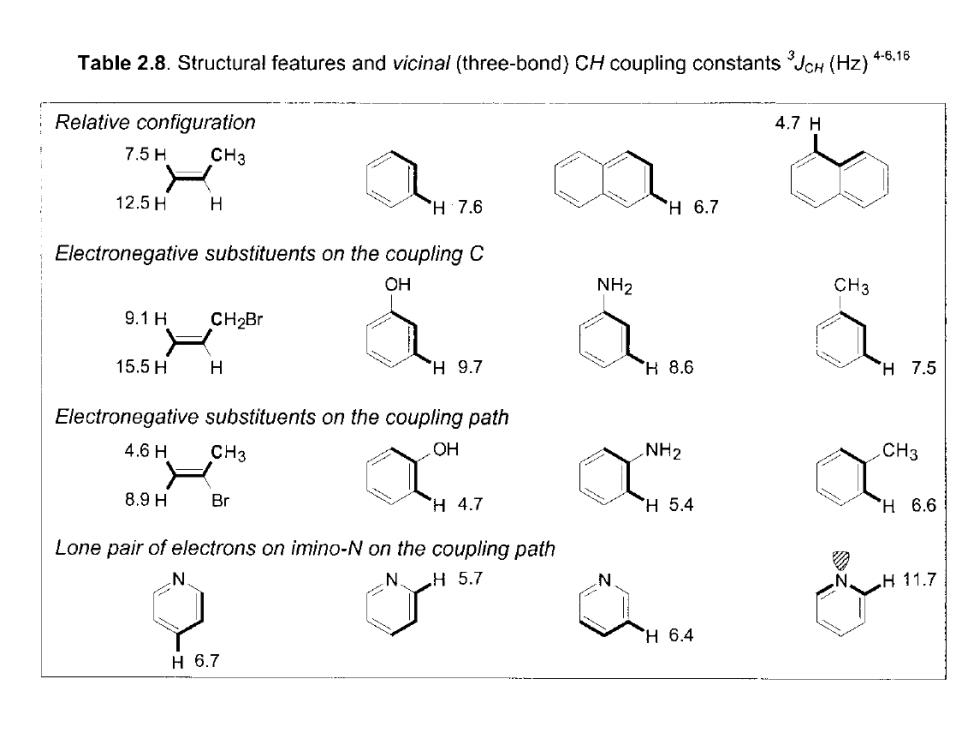
Table 2.8.Structural features and vicinal (three-bond)CH coupling constants(Hz).1 Relative configuration 4.7H 7.5H CH3 12.5HH H7.6 6.7 Electronegative substituents on the coupling C OH NH2 CH3 9.1h CH2Br 15.5H H H97 H8.6 7.5 Electronegative substituents on the coupling path 4.6H CH3 OH NH2 CH3 8.9H Br H 4.7 H5.4 H 6.6 Lone pair of electrons on imino-N on the coupling path H5.7 11.7 H6.4 H6.7