Chapter4.药物的构效关系(SAR) Structure-Activity Relationships -67973 674973 本章目录 L药物结构 Ⅱ.药物的作用靶点(Targets) Ⅲ药物-靶标的分子识别和相互作用 V定量构效关系
Chapter 4. 药物的构效关系(SAR) Structure-Activity Relationships 本章目录 I. 药物结构 II. 药物的作用靶点(Targets) III. 药物-靶标的分子识别和相互作用 IV. 定量构效关系
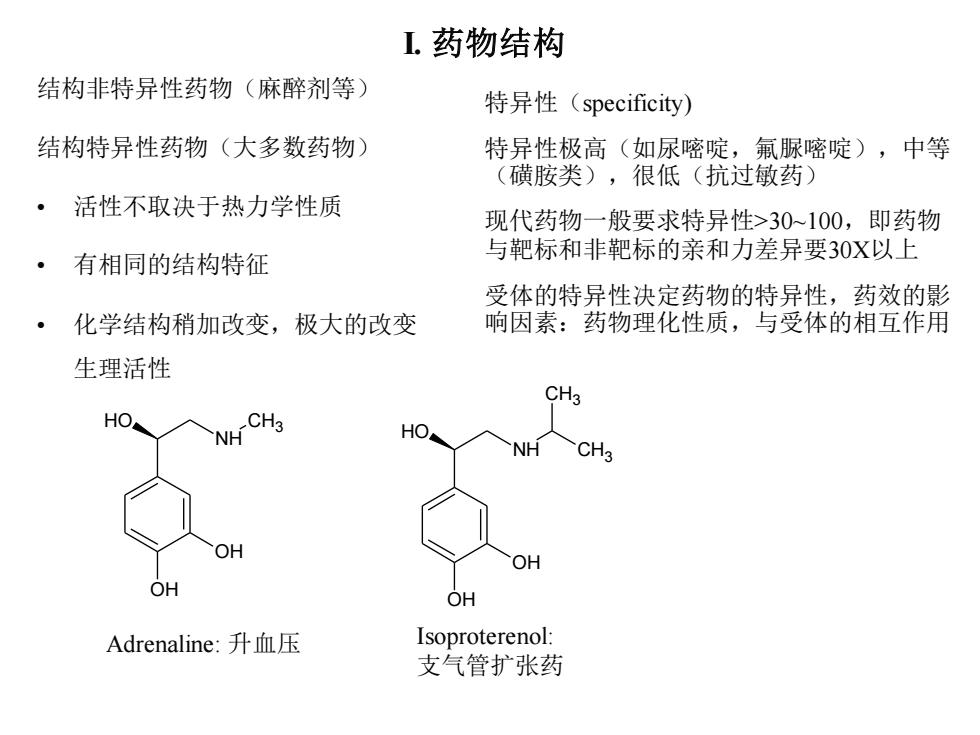
L药物结构 结构非特异性药物(麻醉剂等) 特异性(specificity) 结构特异性药物(大多数药物) 特异性极高(如尿嘧啶,氟脲嘧啶),中等 (磺胺类),很低(抗过敏药) 活性不取决于热力学性质 现代药物一般要求特异性>30~100,即药物 有相同的结构特征 与靶标和非靶标的亲和力差异要30X以上 受体的特异性决定药物的特异性,药效的影 化学结构稍加改变,极大的改变 响因素:药物理化性质,与受体的相互作用 生理活性 CH3 HO NH CH3 HO NH CH3 OH OH OH OH Adrenaline:升血压 Isoproterenol: 支气管扩张药
I. 药物结构 结构非特异性药物(麻醉剂等) 结构特异性药物(大多数药物) • 活性不取决于热力学性质 • 有相同的结构特征 • 化学结构稍加改变,极大的改变 生理活性 OH NH OH HO CH3 OH NH OH HO CH3 CH3 Adrenaline: 升血压 Isoproterenol: 支气管扩张药 特异性(specificity) 特异性极高(如尿嘧啶,氟脲嘧啶),中等 (磺胺类),很低(抗过敏药) 现代药物一般要求特异性>30~100,即药物 与靶标和非靶标的亲和力差异要30X以上 受体的特异性决定药物的特异性,药效的影 响因素:药物理化性质,与受体的相互作用

小分子药物结构解析 General structure elucidation procedure: 1.Determine molecular formula by MS 2.Degree of Unsaturation =[2+(2 x #Carbons)+#Nitrogens- #Hydrogens-#Halogens]/2 3.Acquire 1H,HSQC and HMBC,write down chemical shifts and build connectivity 4.Acquire 1D TOCSY NOESY to resolve ambiguity 5.Draw a tentative structure and check consistency with spectra Note:the presence of peaks can be proof of nuclear and its connectivity the absence of peaks can NOT be proof of no connectivity!
小分子药物结构解析 General structure elucidation procedure: 1. Determine molecular formula by MS 2. Degree of Unsaturation = [2 + (2 x #Carbons) + #Nitrogens - #Hydrogens - #Halogens]/2 3. Acquire 1H, HSQC and HMBC, write down chemical shifts and build connectivity 4. Acquire 1D TOCSY & NOESY to resolve ambiguity 5. Draw a tentative structure and check consistency with spectra Note: the presence of peaks can be proof of nuclear and its connectivity the absence of peaks can NOT be proof of no connectivity!
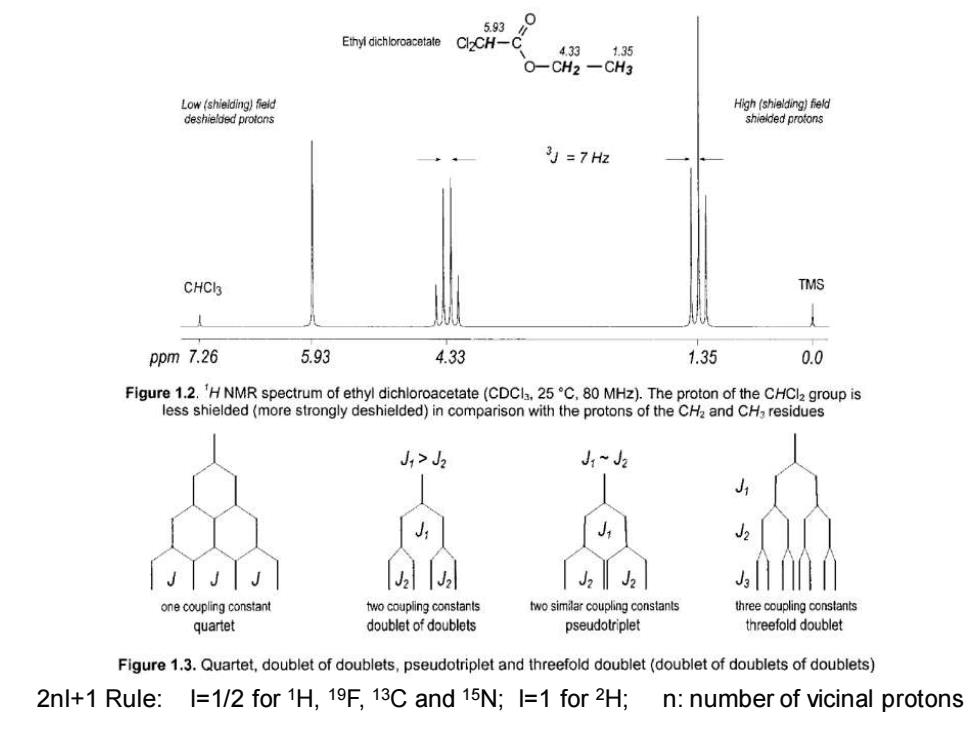
593 Ethyl dichloroacetate Cl2CH-0 4.33.1.35 O-CH2-CH3 Low(shielding)feld High (shielding)field deshielded protons shielded protons 为=7H2 CHCI TMS ppm 7.26 5.93 4.33 1.35 0.0 Figure 1.2.'H NMR spectrum of ethyl dichloroacetate(CDCla.25C.80 MHz).The proton of the CHCI2 group is less shielded(more strongly deshielded)in comparison with the protons of the CH2 and CH,residues ,> 12 3 one coupling constant two coupling constants two similar coupling constants three coupling constants quartet doublet of doublets pseudotriplet threefold doublet Figure 1.3.Quartet,doublet of doublets,pseudotriplet and threefold doublet(doublet of doublets of doublets) 2nl+1 Rule:I=1/2 for 1H,19F,13C and 15N;I=1 for 2H; n:number of vicinal protons
2nI+1 Rule: I=1/2 for 1H, 19F, 13C and 15N; I=1 for 2H; n: number of vicinal protons
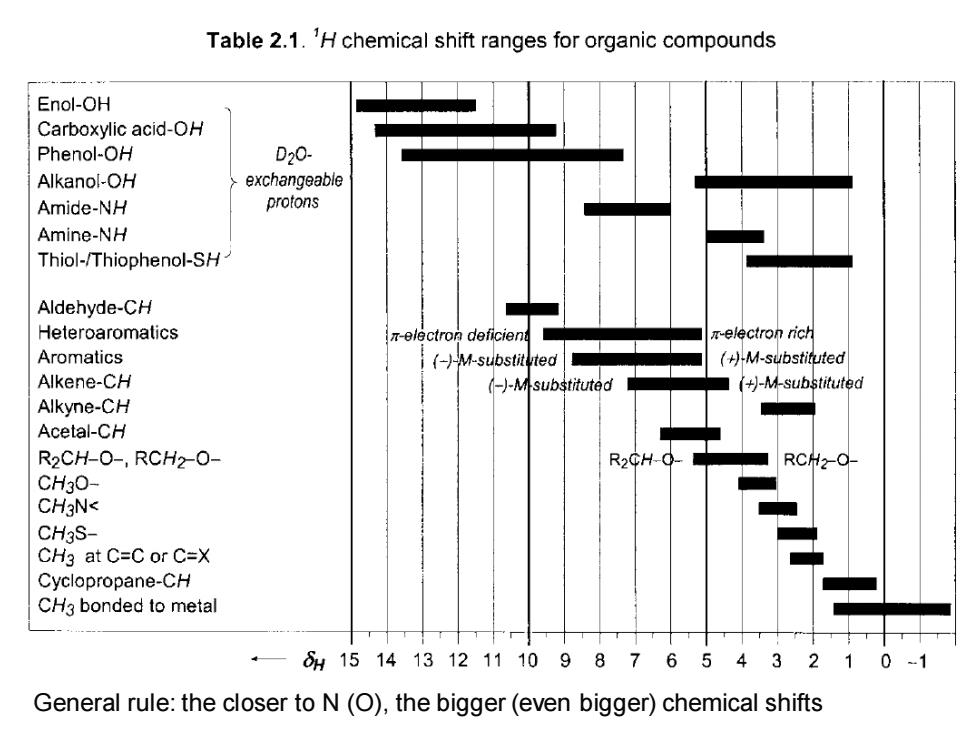
Table 2.1.'H chemical shift ranges for organic compounds Enol-OH Carboxylic acid-OH Phenol-OH D20- Alkanol-OH exchangeable Amide-NH protons Amine-NH Thiol-/Thiophenol-SH Aldehyde-CH Heteroaromatics π-electron deficient -electron rich Aromatics (-)-M-substituted /+月M-substit近uted Alkene-CH (-)-M-substituted (+)-M-substituted Alkyne-CH Acetal-CH R2CH-0-,RCH2-0- R2CH-0- RCH2-O- CH30- CHgN< CH3S- CH3 at C=C or C=X Cyclopropane-CH CH3 bonded to metal ·—61514131211109876543210 General rule:the closer to N(O),the bigger(even bigger)chemical shifts
General rule: the closer to N (O), the bigger (even bigger) chemical shifts