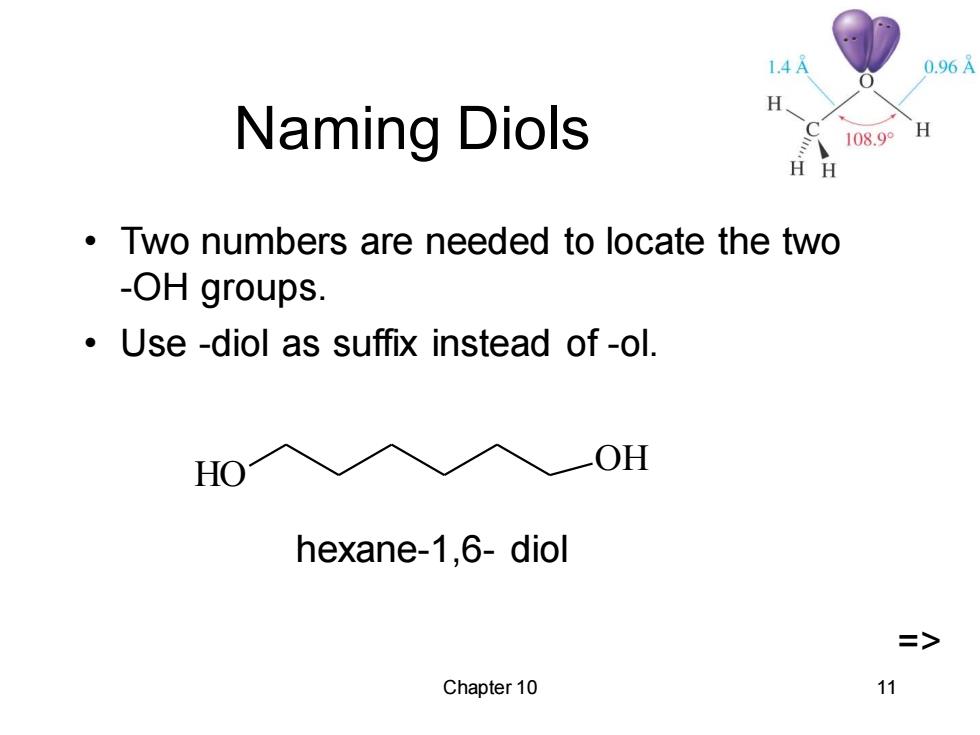
1.4A 0.96A H Naming Diols 108.9° H H H Two numbers are needed to locate the two -OH groups. Use -diol as suffix instead of-ol. HO OH hexane-1,6-diol => Chapter 10 11
Chapter 10 11 Naming Diols • Two numbers are needed to locate the two -OH groups. • Use -diol as suffix instead of -ol. HO OH hexane-1,6- diol =>
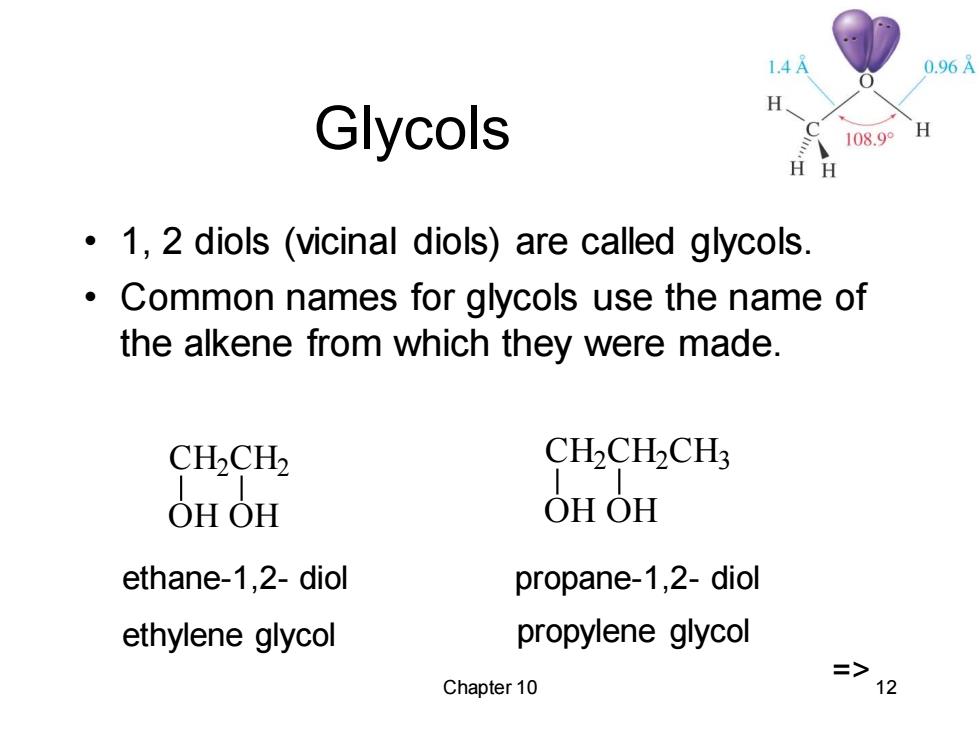
1.4A 0.96A Glycols 108.9° H H 1,2 diols (vicinal diols)are called glycols. Common names for glycols use the name of the alkene from which they were made. CH2CH2 CH2CH2CH3 OHOH OHOH ethane-1,2-diol propane-1,2-diol ethylene glycol propylene glycol Chapter 10 72
Chapter 10 12 Glycols • 1, 2 diols (vicinal diols) are called glycols. • Common names for glycols use the name of the alkene from which they were made. CH2 CH2 OH OH CH2 CH2 CH3 OH OH ethane-1,2- diol ethylene glycol propane-1,2- diol propylene glycol =>
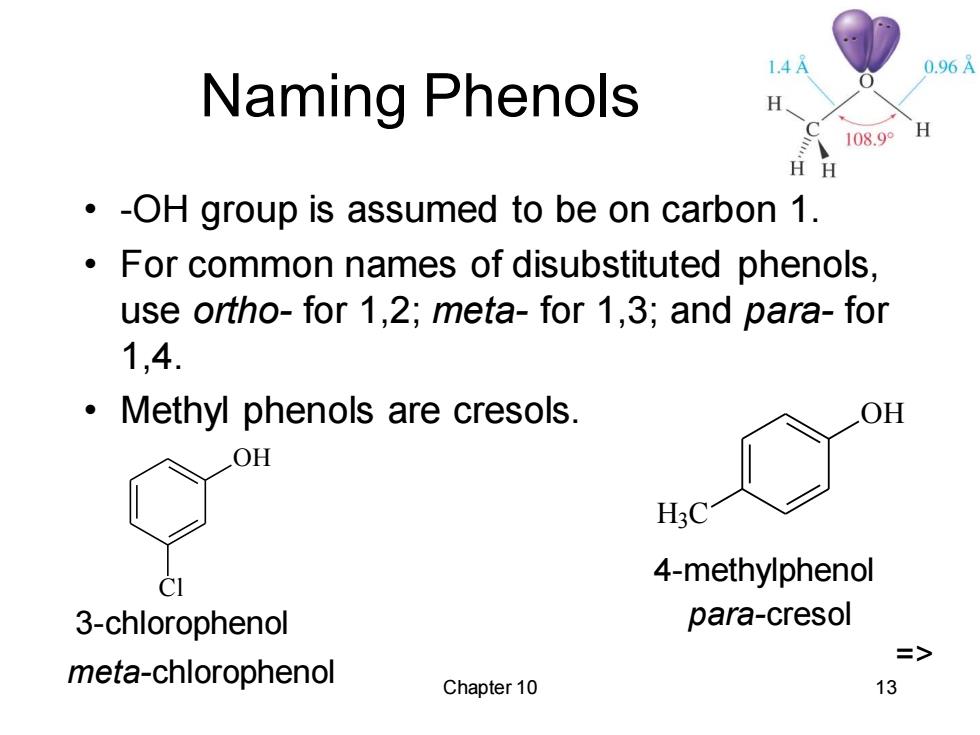
1.4 0.96A Naming Phenols H 108.9 H H -OH group is assumed to be on carbon 1. For common names of disubstituted phenols, use ortho-for 1,2;meta-for 1,3;and para-for 1,4. Methyl phenols are cresols. OH OH H3C 4-methylphenol 3-chlorophenol para-cresol => mefa-chlorophenol Chapter 10 13
Chapter 10 13 Naming Phenols • -OH group is assumed to be on carbon 1. • For common names of disubstituted phenols, use ortho- for 1,2; meta- for 1,3; and para- for 1,4. • Methyl phenols are cresols. OH Cl 3-chlorophenol meta-chlorophenol OH H3 C 4-methylphenol para-cresol =>

1.4 0.96A Physical Properties 108.9° H H Unusually high boiling points due to hydrogen bonding between molecules. Small alcohols are miscible in water,but solubility decreases as the size of the alkyl group increases. => Chapter 10 14
Chapter 10 14 Physical Properties • Unusually high boiling points due to hydrogen bonding between molecules. • Small alcohols are miscible in water, but solubility decreases as the size of the alkyl group increases. =>
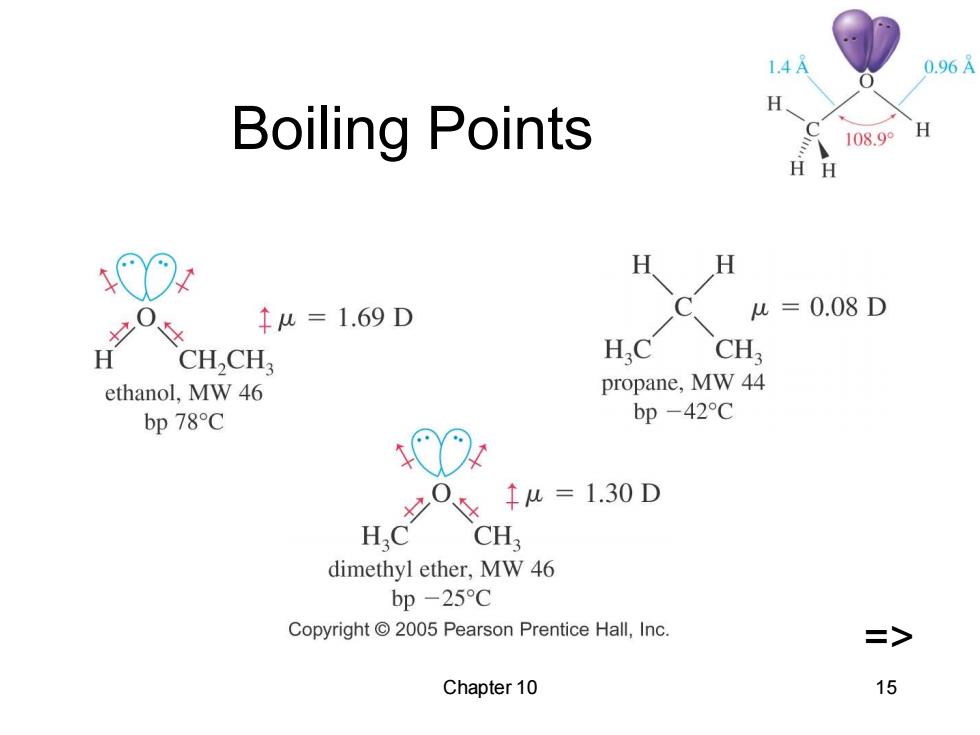
1.4 0.96 Boiling Points 108.9° H H H O H ↑u=1.69D =0.08D H CH,CH HC CH ethanol,MW 46 propane,MW 44 bp 78C bp-42C 00 0 ↑u=1.30D HC CH dimethyl ether,MW 46 bp -25C Copyright 2005 Pearson Prentice Hall,Inc. => Chapter 10 15
Chapter 10 15 Boiling Points =>