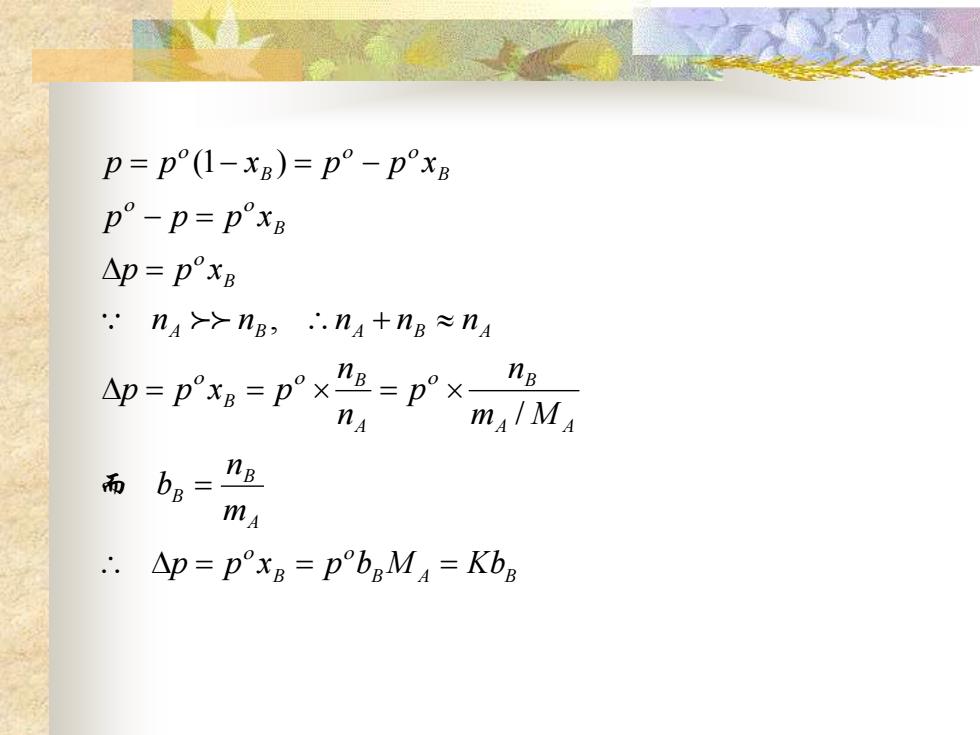
p=p(1-xB)=p°-pxB p°-p=pxB △p=pxB .'nA>->nB,∴.nA+nB≈nA p=p°xB=p° ×nB=p× nB mA/MA 而 b。=g mA ∴.△p=pxB=pbgMA=KbB
B B A B A B B A A B A B B A B A B A B B B B p p x p b M Kb m n b m M n p n n p p x p n n n n n p p x p p p x p p x p p x = = = = = = = + = − = = − = − 而 / , (1 )

例已知293K时水的饱和蒸汽压为2.338kPa,将6.840g蔗糖 (C12H2201)溶于100.0g水中,计算蔗糖溶液的质量摩尔浓度 和蒸汽压。 be= 6.840g 342.0g.mol- 1000gkg=0.2000 mol-kg 100.0g XA 100.0g18.02gmol- 100.0g18.02gmol+6.840g342.0gmol 55.49mol =0.9964 (55.49+0.02000)mol p=pxa=2.338kPa×0.9964=2.330kPa
例 已知293K时水的饱和蒸汽压为2.338 kPa,将6.840 g蔗糖 (C12H22O11)溶于100.0 g水中,计算蔗糖溶液的质量摩尔浓度 和蒸汽压 。 p = p 0xA = 2.338 kPa×0.996 4 = 2.330 kPa -1 -1 B -1 0.2000 mol kg 100.0 g 1000 g kg 342.0 g mol 6.840 g = b = 0.996 4 (55.49 0.02000)mol 55.49mol 100.0 g/18.02 g mol 6.840g/342.0g mol 100.0 g/18.02 g mol -1 1 -1 A = + = + = − x