
Glossary Molecular ion -The ion obtained by the loss of one electron from the molecule (m) Base peak-The most intense peak in the MS, assigned 100%intensity Radical cation-positively charged species with an odd number of electrons Fragment ions-Lighter cations (and radical cations)formed by the decomposition of the molecular ion.These often correspond to stable carbcations. .m/z-mass to charge ratio
Glossary • Molecular ion - The ion obtained by the loss of one electron from the molecule (m+ ) • Base peak - The most intense peak in the MS, assigned 100% intensity • Radical cation - positively charged species with an odd number of electrons • Fragment ions - Lighter cations (and radical cations) formed by the decomposition of the molecular ion. These often correspond to stable carbcations. • m/z - mass to charge ratio
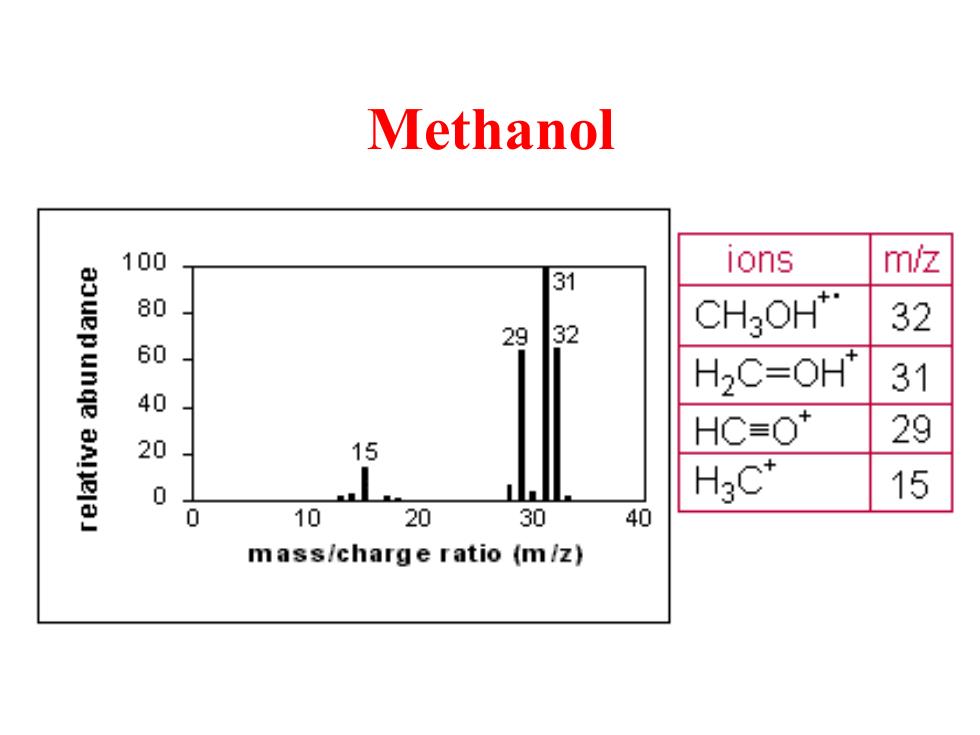
Methanol 100 ions m/z 31 aouepunge 80 29 32 CH3OH* 32 60 H2C=0H* 31 40 20 HC≡O 29 15 0 H3C* 15 0 10 20 30 40 mass/charge ratio (m/z)
Methanol
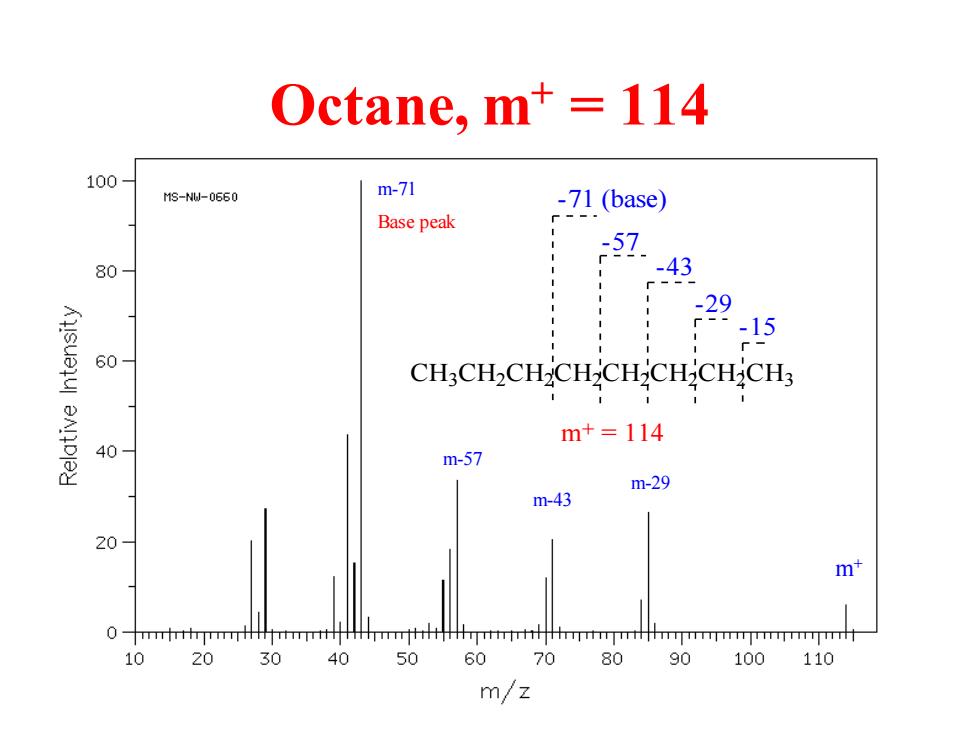
Octane,m+114 100 1s-N1-0560 m-71 -71(base) Base peak - 57 80 -43 -29 -15 60 CH:CH2CH-CH-CH-CH-CH2CH3 m+=114 40 m-57 m-29 m-43 20 m+ 0 -rrmmmllmrHrmhhpmtmmhmmmmpmr 10 20 30 40 50 60 70 80 90 100 110 m/z
Octane, m+ = 114 CH3CH2CH2CH2CH2CH2CH2CH3 m+ = 114 -15 -29 -43 -57 -71 (base) m-29 m-43 m-57 m-71 Base peak m+
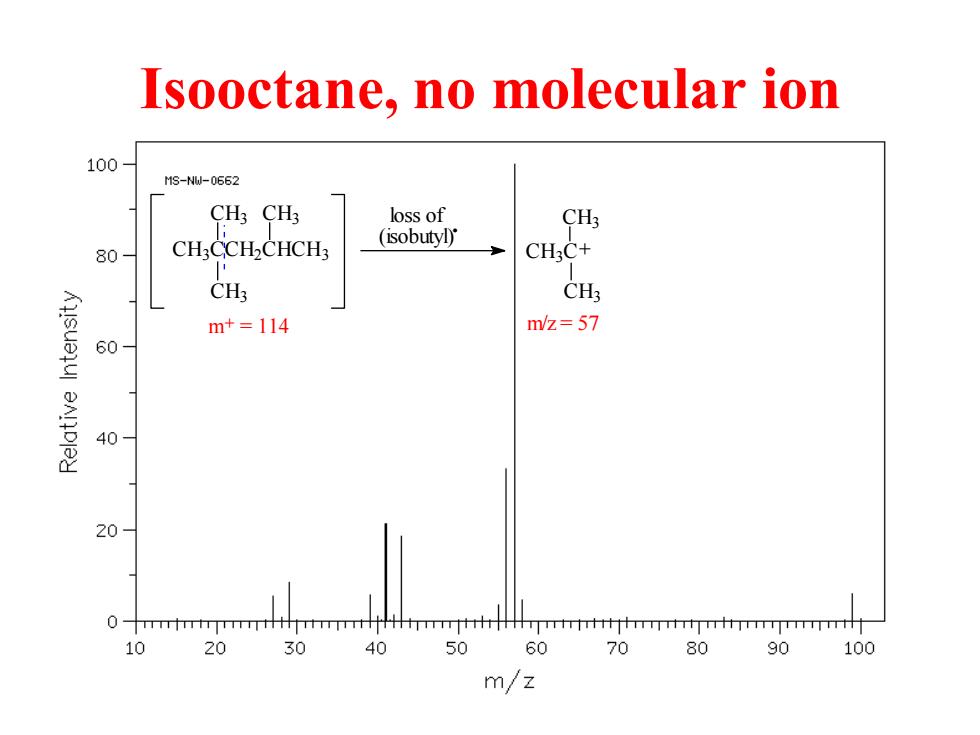
Isooctane,no molecular ion 100 M3-NW-0662 CH3CH3 loss of CH3 CH:CCH2CHCH3 (isobutyl) 80 CH3C+ CHs CH3 m+=114 m/z=57 60 BAlDley 40 20 10 20 30 40 50 60 70 80 90 100 m/z
Isooctane, no molecular ion CH3CCH2CHCH3 CH3 CH3 CH3 m+ = 114 loss of (isobutyl). CH3C CH3 CH3 + m/z = 57
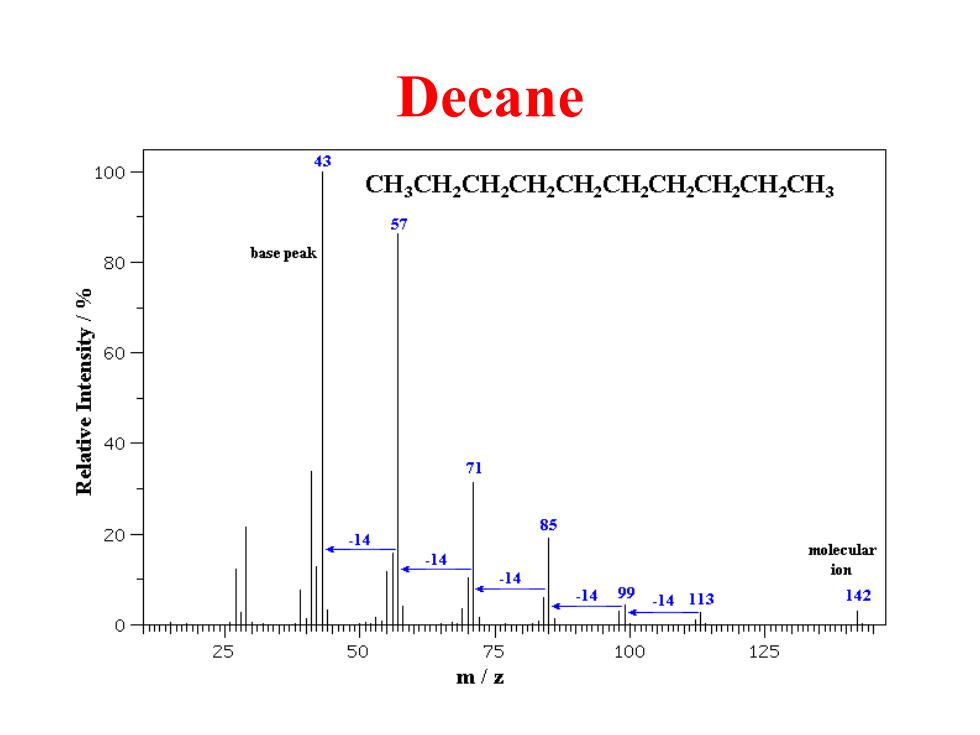
Decane 43 100- CH.CH.CH.CH.CH.CH.CH.CH.CHCH 57 80 base peak 3 HSUu 60 SAREPH 40 71 85 20 -14 -14 molecular -14 ion 149914113 142 0-mmmtmirmmmmimmtnmmmhmmmjrmmmnmmmmjmmmmmmmim 25 50 75 100 125 m/z
Decane