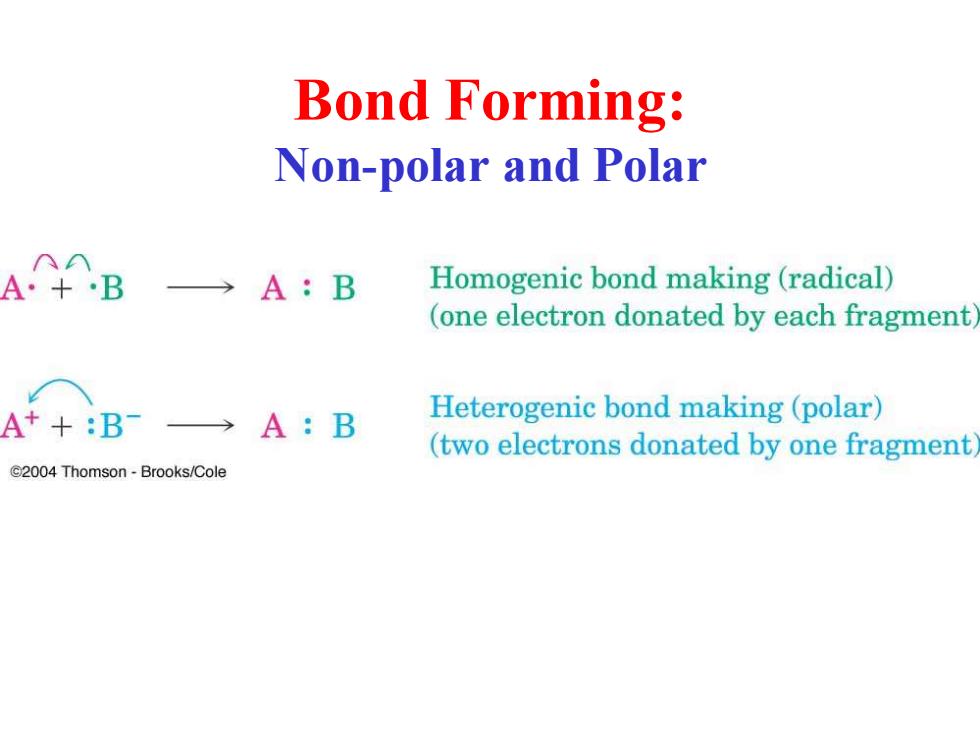
Bond Forming: Non-polar and Polar A·+B →A:B Homogenic bond making (radical) (one electron donated by each fragment A++:B→A:B Heterogenic bond making(polar) (two electrons donated by one fragment C2004 Thomson-Brooks/Cole
Bond Forming: Non-polar and Polar
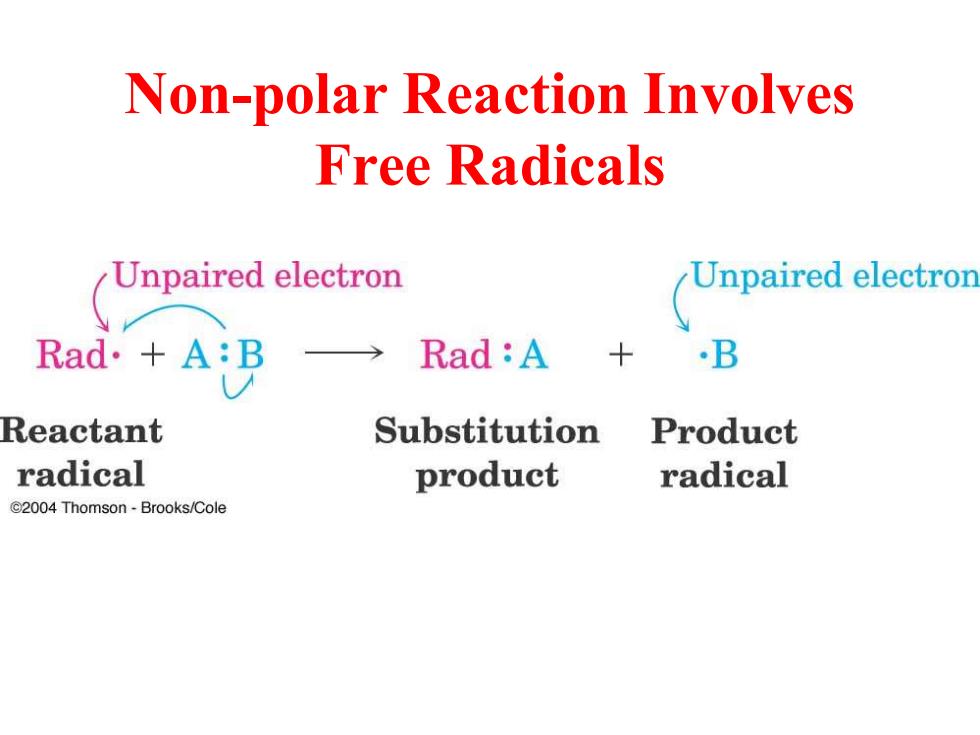
Non-polar Reaction Involves Free Radicals Unpaired electron Unpaired electron Rad.+A:B →Rad:A B Reactant Substitution Product radical product radical e2004 Thomson-Brooks/Cole
Non-polar Reaction Involves Free Radicals
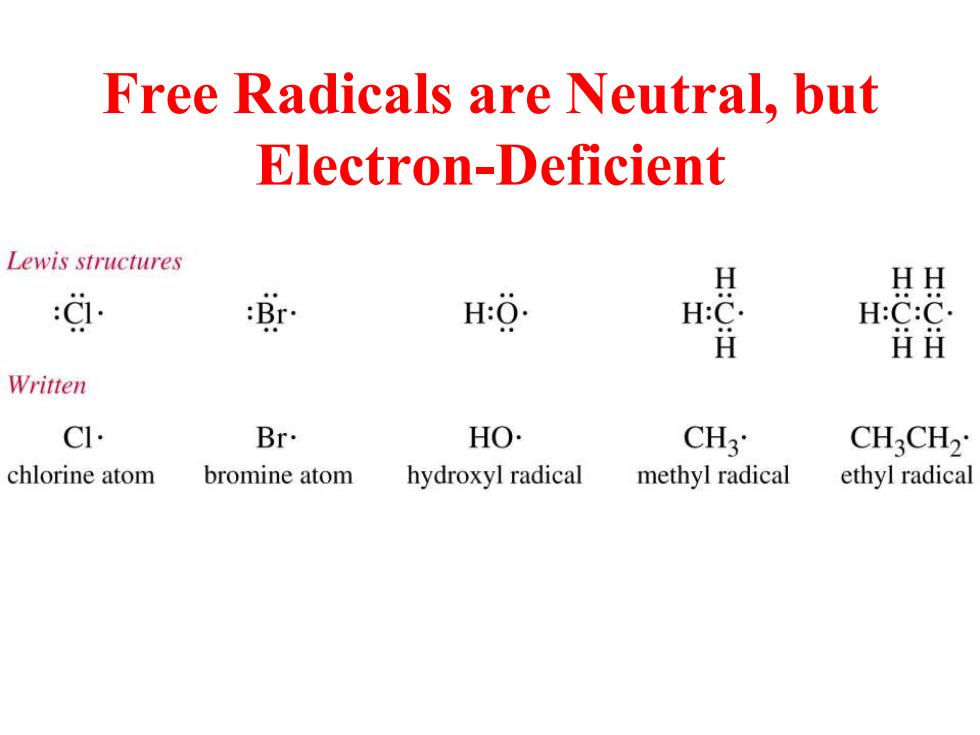
Free Radicals are Neutral,but Electron-Deficient Lewis structures HH 9. 海 H H:O H:C. H:C:C. ǚⅱ Written CI. Br. HO. CH3 CH3CH2 chlorine atom bromine atom hydroxyl radical methyl radical ethyl radical
Free Radicals are Neutral, but Electron-Deficient
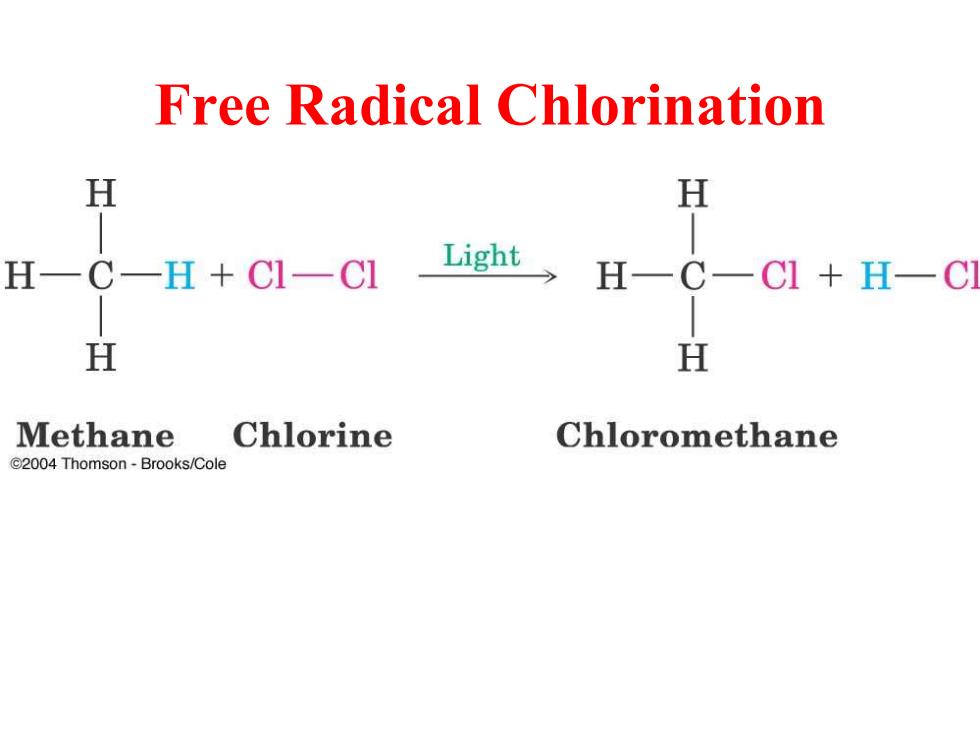
Free Radical Chlorination H H H一CH+C-C Light H一C一CI+H-C H H Methane Chlorine Chloromethane e2004 Thomson-Brooks/Cole
Free Radical Chlorination

Experimental Evidence Helps to Determine Mechanism Chlorination does not occur at room temperature in the dark. The most effective wavelength of light is blue that is strongly absorbed by Cl2 gas. The light-initiated reaction has a high quantum yield (many molecules of product are formed from each photon of light) C photon (v) :C+C:
Experimental Evidence Helps to Determine Mechanism • Chlorination does not occur at room temperature in the dark. • The most effective wavelength of light is blue that is strongly absorbed by Cl2 gas. • The light-initiated reaction has a high quantum yield (many molecules of product are formed from each photon of light)