
Process and Path Type of process: (1)Constant temperature process:T-T2=Text (2)Constant pressure process:P=P2=Pext (3)Constant volume process:V=V2 (4)Cycle process:initial state final state (5)Adiabatic process:Q=0 (6)Constant exterior pressure process:Pext=constant (7)Expansion against vacuum (Free expansion):Pext=0 PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint,com,cn
Process and Path Type of process: (1) Constant temperature process: T1 =T2 =Text (2) Constant pressure process: P1 =P2 =Pext (3) Constant volume process: V1 =V2 (4) Cycle process: initial state = final state (5) Adiabatic process: Q=0 (6) Constant exterior pressure process: Pext =constant (7) Expansion against vacuum (Free expansion): Pext=0 PDF 文件使用 "pdfFactory Pro" 试用版本创建 f www.fineprint.com.cn ÿ
Heat and Work .The two forms of energy transfer are: -Heat(Q):difference in temperature between system and surroundings. -Work(w):the energy transferred when an object is moved by a force. PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
Heat and Work •The two forms of energy transfer are: §Heat (Q): difference in temperature between system and surroundings. §Work (w): the energy transferred when an object is moved by a force. PDF 文件使用 "pdfFactory Pro" 试用版本创建 f www.fineprint.com.cn ÿ
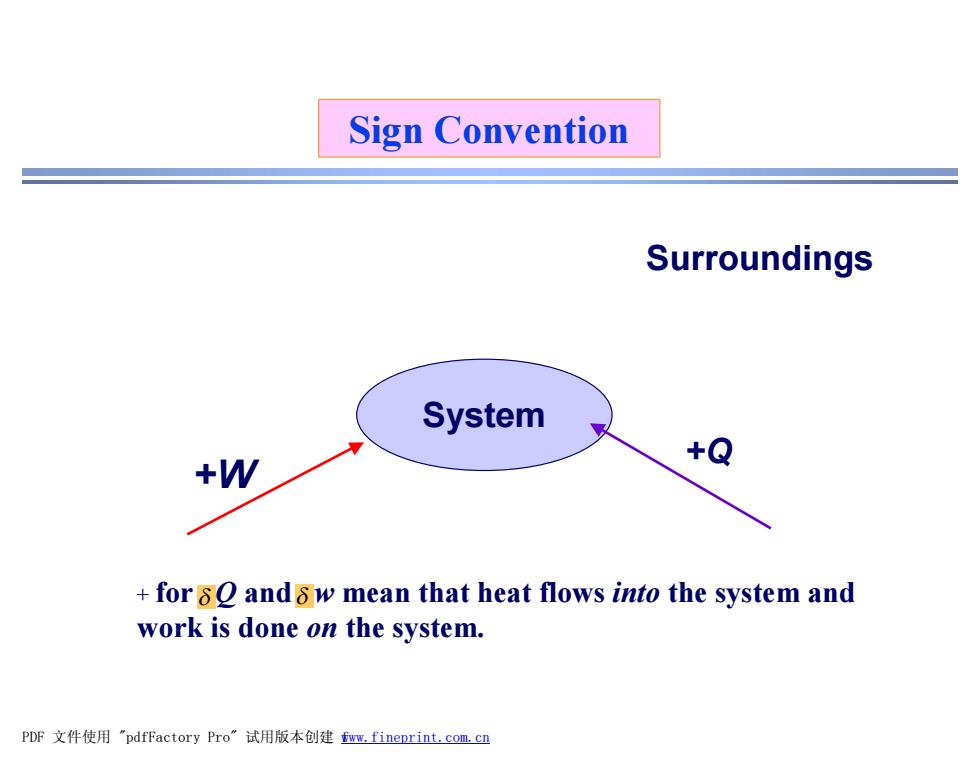
Sign Convention Surroundings System +W +Q for 8O and8w mean that heat flows into the system and work is done on the system. PDF文件使用"pdfFactory Pro”试用版本创建fmm,fineprint.com,cn
Sign Convention System Surroundings +W +Q + for Q and w mean that heat flows into the system and work is done on the system. d d PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn
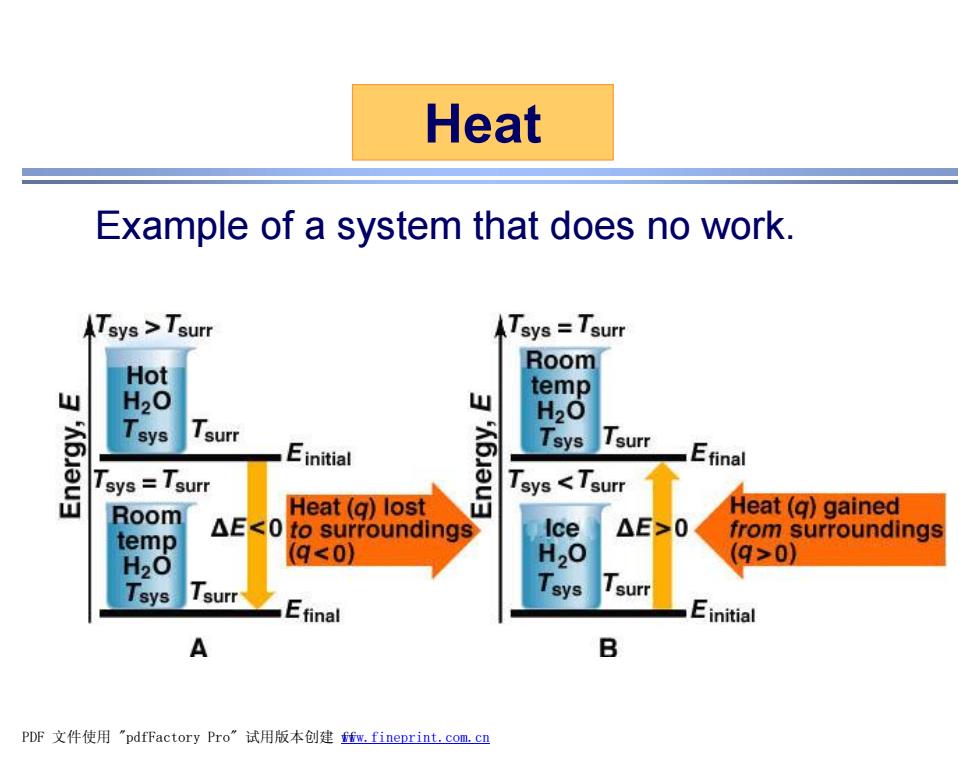
Heat Example of a system that does no work. Tsys >Tsurr Tsys=Tsurr Room Hot temp 山 H20 H2O Tsys Tsurr Einitial Tsys Tsurr Efinal 盖 Tsys=Tsurr Tsys <Tsurr Room Heat(q)lost Heat(q)gained Ice △E>0 temp △E<0 to surroundings from surroundings H20 (q<0)】 H,O q>0) Tsys Isurr Tsys I surr Efinal Einitial A B PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
Heat Example of a system that does no work. PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn f

Work .An eg.of a system where energy is transferred solely by work,Q=0 as the system is insulated. .w<0 as the volume change System occurs against an external pressure. HCI(ag) Zng+2HCl(a)→H2g+ Zn(s) ZnCl2(aq) Einitial .The work is performed by the H2(g) Work (w)done on .This kind of work is called System AE<0 H2(g) surroundings(w<0) pressure volume work,(PV work). ZnCl2(aq) Efinal PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint..com,cn
Work •An eg. of a system where energy is transferred solely by work, Q = 0 as the system is insulated. •w < 0 as the volume change occurs against an external pressure. •Zn(s) + 2HCl (aq) ® H2(g) + ZnCl2(aq) •The work is performed by the H2(g). •This kind of work is called pressure volume work, (PV work). PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn f