
Chapter 9 Ultraviolet Spectrophotometry, UV (紫外吸收光谱分析)
Chapter 9 Ultraviolet Spectrophotometry, UV (紫外吸收光谱分析)
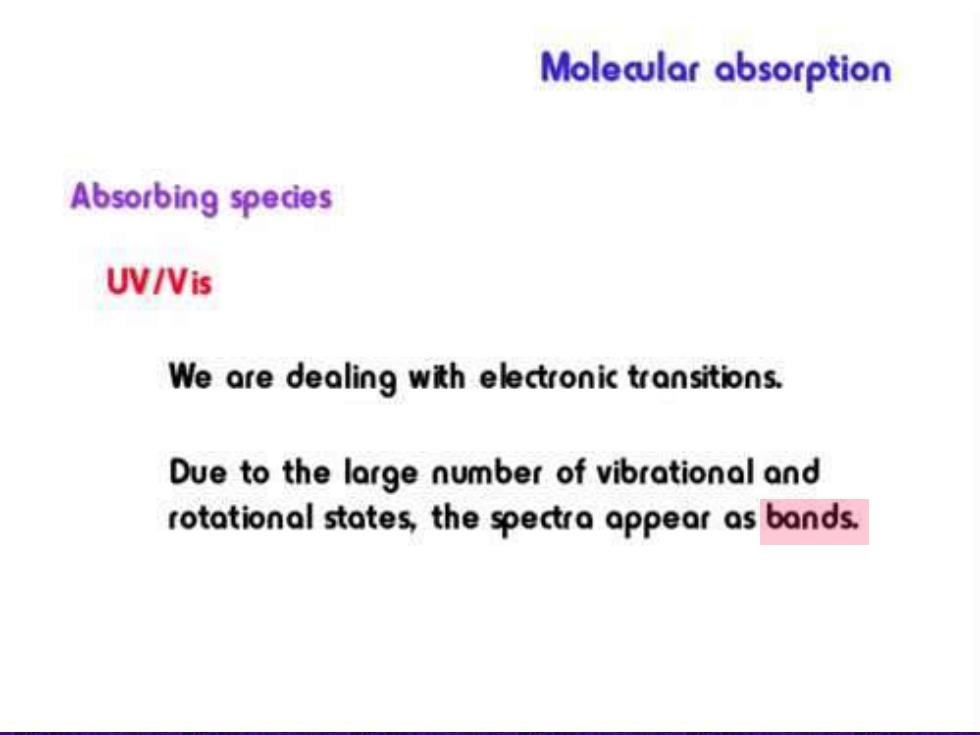
Moleaular absorption Absorbing species UV/Vis We are dealing with electronic transitions. Due to the large number of vibrational and rotational states,the spectra appear as bands
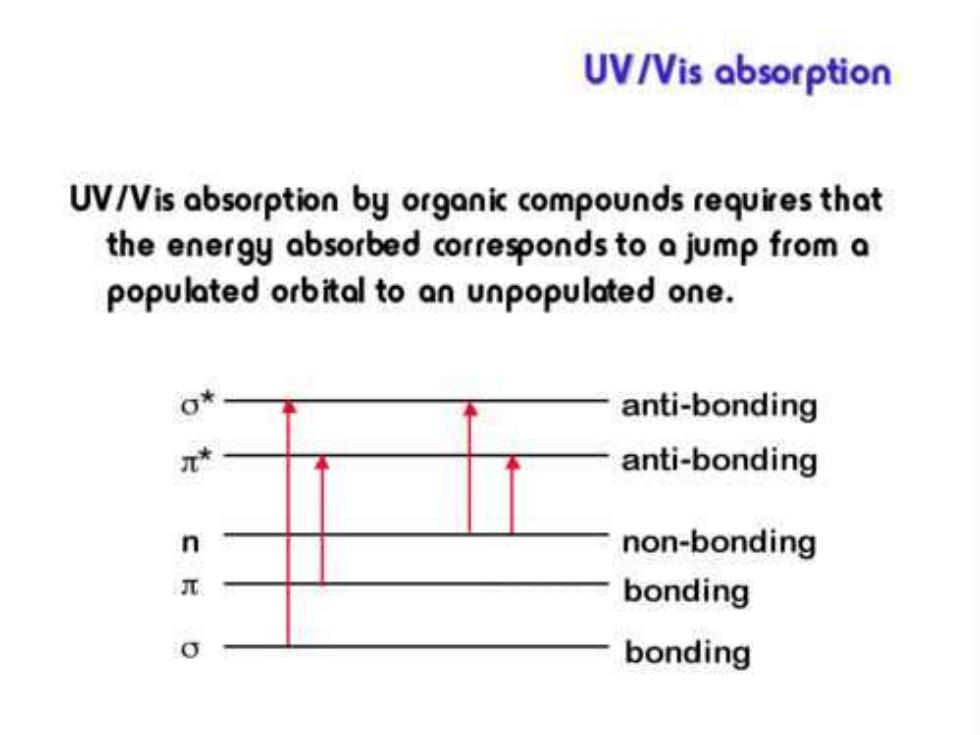
UV/Vis absorption UV/Vis absorption by organic compounds requires that the energy absorbed corresponds to a jump from a populated orbital to an unpopulated one. anti-bonding anti-bonding n non-bonding bonding bonding

Electronic transitions include three kinds of types: ● N→V transitions include →* transitions in saturated organic compounds andππ* transitions in unsaturated compounds. ● N→Q transitions: a kind of transitions arise from the electron excitation from nonbonding orbital to anti-bonding orbital. ● N→R transition: electron is excited to a higher level until it is ionized to molecular ion. ● Charge transfer transitions: charge (electron) transfers between different parts of the compound due to charge redistribution of the compound excited by a radiation
Electronic transitions include three kinds of types: ● N→V transitions include →* transitions in saturated organic compounds andππ* transitions in unsaturated compounds. ● N→Q transitions: a kind of transitions arise from the electron excitation from nonbonding orbital to anti-bonding orbital. ● N→R transition: electron is excited to a higher level until it is ionized to molecular ion. ● Charge transfer transitions: charge (electron) transfers between different parts of the compound due to charge redistribution of the compound excited by a radiation

* : anti-bond orbital
* : anti-bond orbital