
UV/Vis absorption n->o*transitions The compound must contain atoms with unshared electron pairs. Compounds containing O,S,N and halogens can absorb via this type of transition. Absorptions are typically in the 150-250 nm region and are not very intense
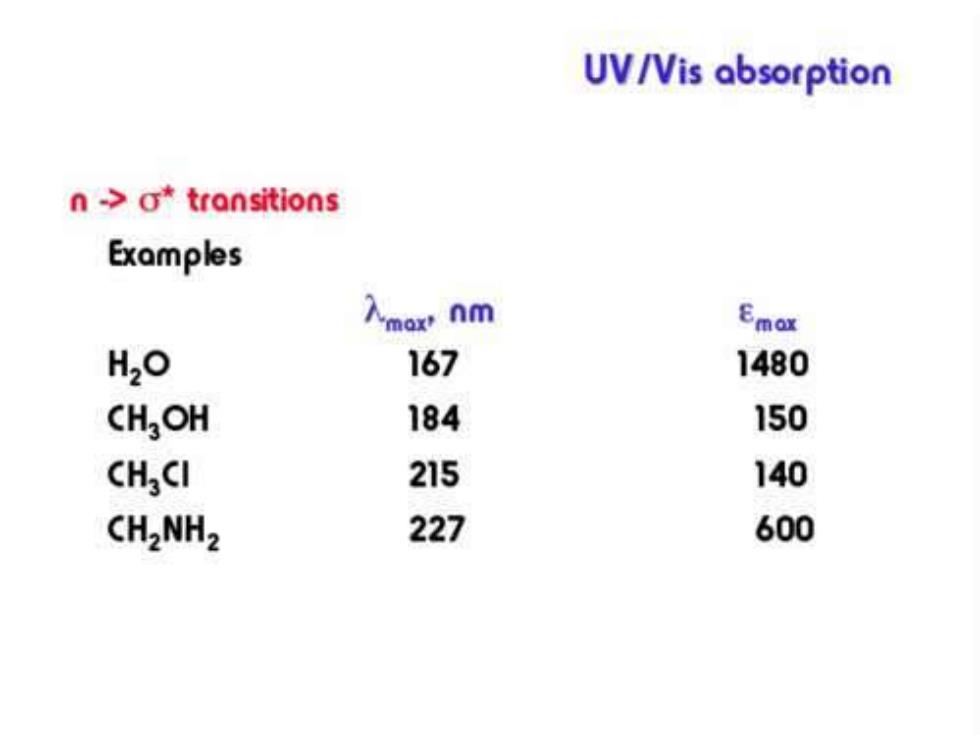
UV/Vis absorption n ->o*transitions Examples 入mox nm Emox H2O 167 1480 CHOH 184 150 CHCI 215 140 CH2NH2 227 600

UV/Vis absorption n->*and>*transitions For a orbital to be available,there must be some degree of unsaturation. multiple bonds and resonance structures These result in some of the most intense absorptions (200-700 nm region). As the degree of unsaturation increases,you typically see a shift to higher
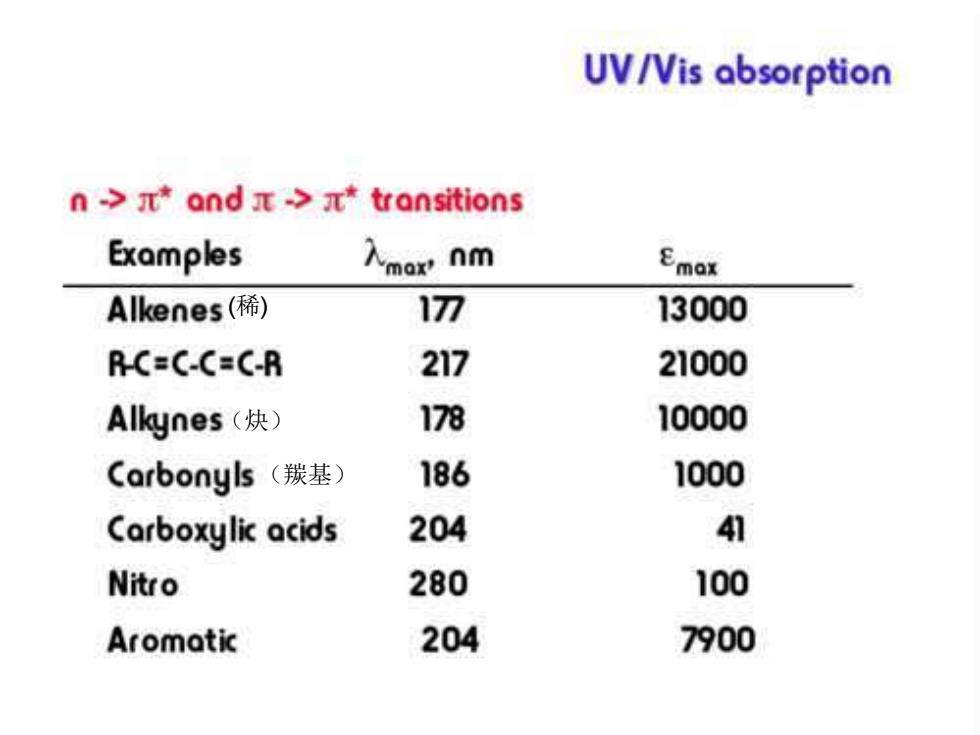
(稀) (炔) (羰基)
(稀) (炔) (羰基)
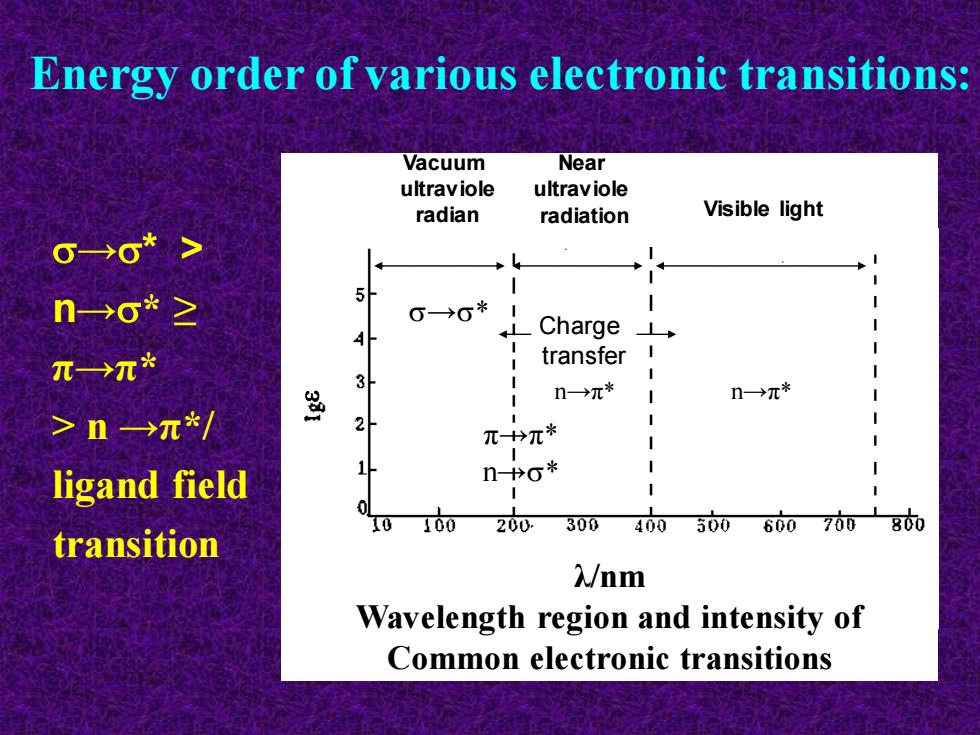
→* > n→* ≥ π→π* > n →π*/ ligand field transition Energy order of various electronic transitions: Vacuum ultraviole radian Near ultraviole radiation Visible light →* π→π* n→* n→π* n→π* Charge transfer λ/nm Wavelength region and intensity of Common electronic transitions
→* > n→* ≥ π→π* > n →π*/ ligand field transition Energy order of various electronic transitions: Vacuum ultraviole radian Near ultraviole radiation Visible light →* π→π* n→* n→π* n→π* Charge transfer λ/nm Wavelength region and intensity of Common electronic transitions