
Chapter 6 Coulometry Instrumental analysis Prof. Yang Wu
Chapter 6 Coulometry Instrumental analysis Prof. Yang Wu

Outline • Faradaic electrolysis law & summary of Coulometry • Principle and equipment of controlled potential Coulometry • Principle and equipment setup of constant current Coulometric titration • Advantages and applications of Coulometric titration • Equipment of auto-Coulometric titration
Outline • Faradaic electrolysis law & summary of Coulometry • Principle and equipment of controlled potential Coulometry • Principle and equipment setup of constant current Coulometric titration • Advantages and applications of Coulometric titration • Equipment of auto-Coulometric titration
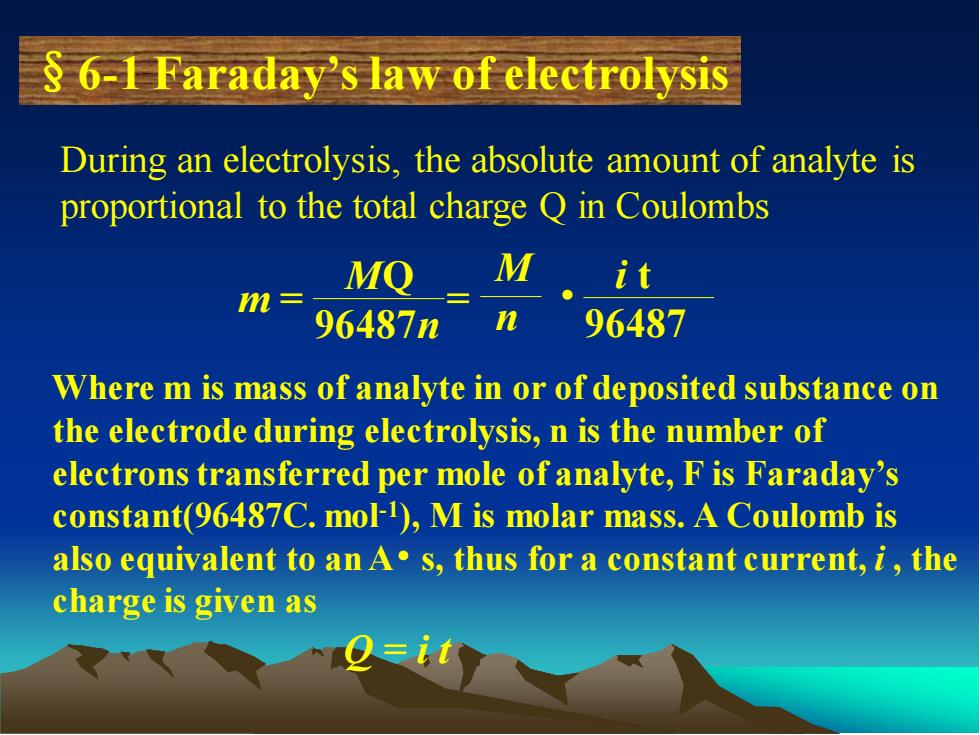
§6-1 Faraday’s law of electrolysis During an electrolysis, the absolute amount of analyte is proportional to the total charge Q in Coulombs m = = MQ 96487n M n i t 96487 • Where m is mass of analyte in or of deposited substance on the electrode during electrolysis, n is the number of electrons transferred per mole of analyte, F is Faraday’s constant(96487C. mol-1 ), M is molar mass. A Coulomb is also equivalent to an A• s, thus for a constant current, i , the charge is given as Q = i t
§6-1 Faraday’s law of electrolysis During an electrolysis, the absolute amount of analyte is proportional to the total charge Q in Coulombs m = = MQ 96487n M n i t 96487 • Where m is mass of analyte in or of deposited substance on the electrode during electrolysis, n is the number of electrons transferred per mole of analyte, F is Faraday’s constant(96487C. mol-1 ), M is molar mass. A Coulomb is also equivalent to an A• s, thus for a constant current, i , the charge is given as Q = i t
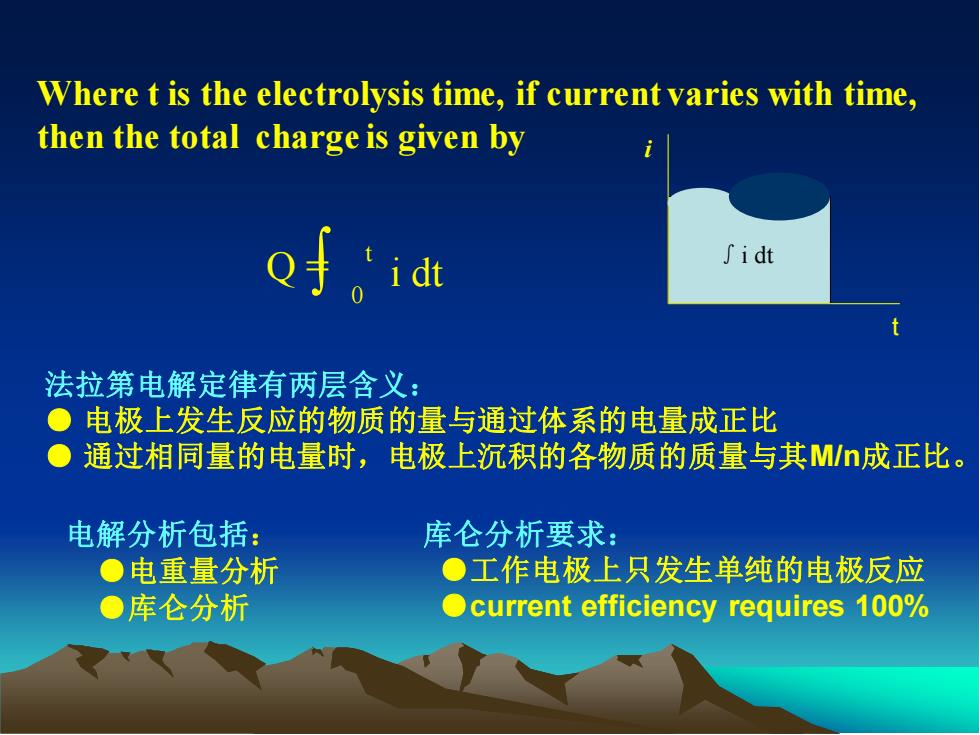
Where t is the electrolysis time, if current varies with time, then the total charge is given by Q =∫ i dt 0 t ∫i dt i t 法拉第电解定律有两层含义: ● 电极上发生反应的物质的量与通过体系的电量成正比 ● 通过相同量的电量时,电极上沉积的各物质的质量与其M/n成正比。 电解分析包括: ●电重量分析 ●库仑分析 库仑分析要求: ●工作电极上只发生单纯的电极反应 ●current efficiency requires 100%
Where t is the electrolysis time, if current varies with time, then the total charge is given by Q =∫ i dt 0 t ∫i dt i t 法拉第电解定律有两层含义: ● 电极上发生反应的物质的量与通过体系的电量成正比 ● 通过相同量的电量时,电极上沉积的各物质的质量与其M/n成正比。 电解分析包括: ●电重量分析 ●库仑分析 库仑分析要求: ●工作电极上只发生单纯的电极反应 ●current efficiency requires 100%
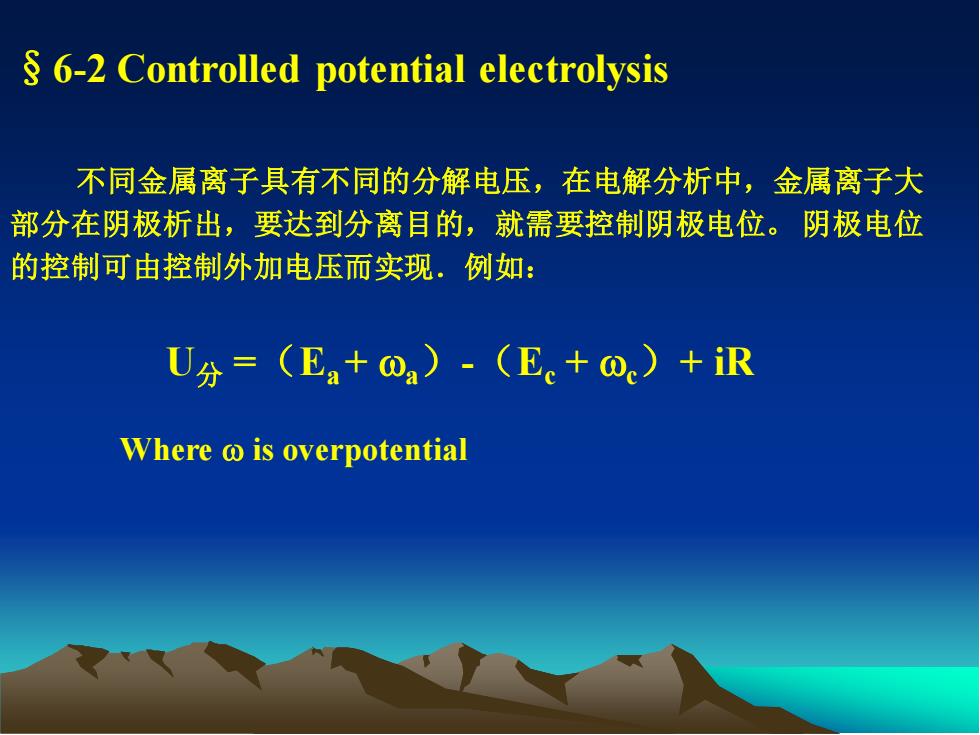
§6-2 Controlled potential electrolysis 不同金属离子具有不同的分解电压,在电解分析中,金属离子大 部分在阴极析出,要达到分离目的,就需要控制阴极电位。 阴极电位 的控制可由控制外加电压而实现.例如: U分 =(Ea + wa)-(Ec + wc)+ iR Where w is overpotential
§6-2 Controlled potential electrolysis 不同金属离子具有不同的分解电压,在电解分析中,金属离子大 部分在阴极析出,要达到分离目的,就需要控制阴极电位。 阴极电位 的控制可由控制外加电压而实现.例如: U分 =(Ea + wa)-(Ec + wc)+ iR Where w is overpotential