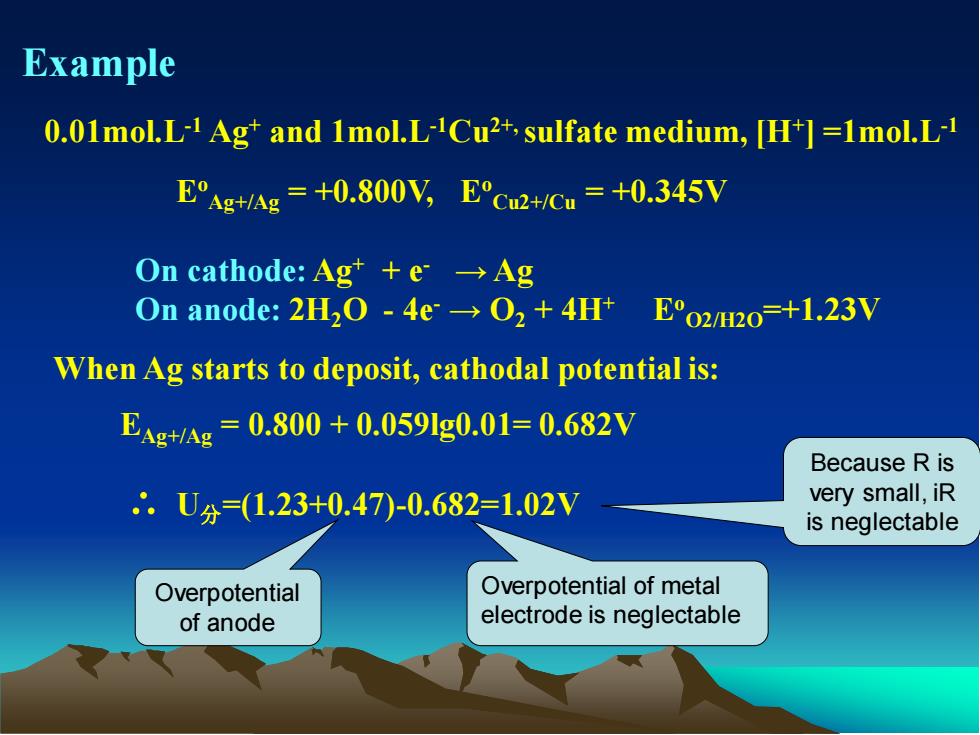
Example 0.01mol.L-1 Ag+ and 1mol.L-1Cu2+, sulfate medium, [H+ ] =1mol.L-1 Eo Ag+/Ag = +0.800V, Eo Cu2+/Cu = +0.345V On cathode: Ag+ + e- → Ag On anode: 2H2O - 4e- → O2 + 4H+ Eo O2/H2O=+1.23V When Ag starts to deposit, cathodal potential is: EAg+/Ag = 0.800 + 0.059lg0.01= 0.682V ∴ U分=(1.23+0.47)-0.682=1.02V Overpotential of anode Overpotential of metal electrode is neglectable Because R is very small, iR is neglectable
Example 0.01mol.L-1 Ag+ and 1mol.L-1Cu2+, sulfate medium, [H+ ] =1mol.L-1 Eo Ag+/Ag = +0.800V, Eo Cu2+/Cu = +0.345V On cathode: Ag+ + e- → Ag On anode: 2H2O - 4e- → O2 + 4H+ Eo O2/H2O=+1.23V When Ag starts to deposit, cathodal potential is: EAg+/Ag = 0.800 + 0.059lg0.01= 0.682V ∴ U分=(1.23+0.47)-0.682=1.02V Overpotential of anode Overpotential of metal electrode is neglectable Because R is very small, iR is neglectable

In fact, as the electrode reaction progresses, applied voltage will be changeable, it can be seen from following result When [Ag+ ] reduces to 10-7mol.L-1 , cathodal potential is EAg+/Ag = 0.800+0.059lg(1×10-7 ) = 0.386V U分= 1.23+0.47 – 0.386=1.31V 此时,Cu2+尚未开始析出(Why?)。可见通过控制外加电位, 可以实现Ag+, Cu2+分离 控制电位电解法
In fact, as the electrode reaction progresses, applied voltage will be changeable, it can be seen from following result When [Ag+ ] reduces to 10-7mol.L-1 , cathodal potential is EAg+/Ag = 0.800+0.059lg(1×10-7 ) = 0.386V U分= 1.23+0.47 – 0.386=1.31V 此时,Cu2+尚未开始析出(Why?)。可见通过控制外加电位, 可以实现Ag+, Cu2+分离 控制电位电解法