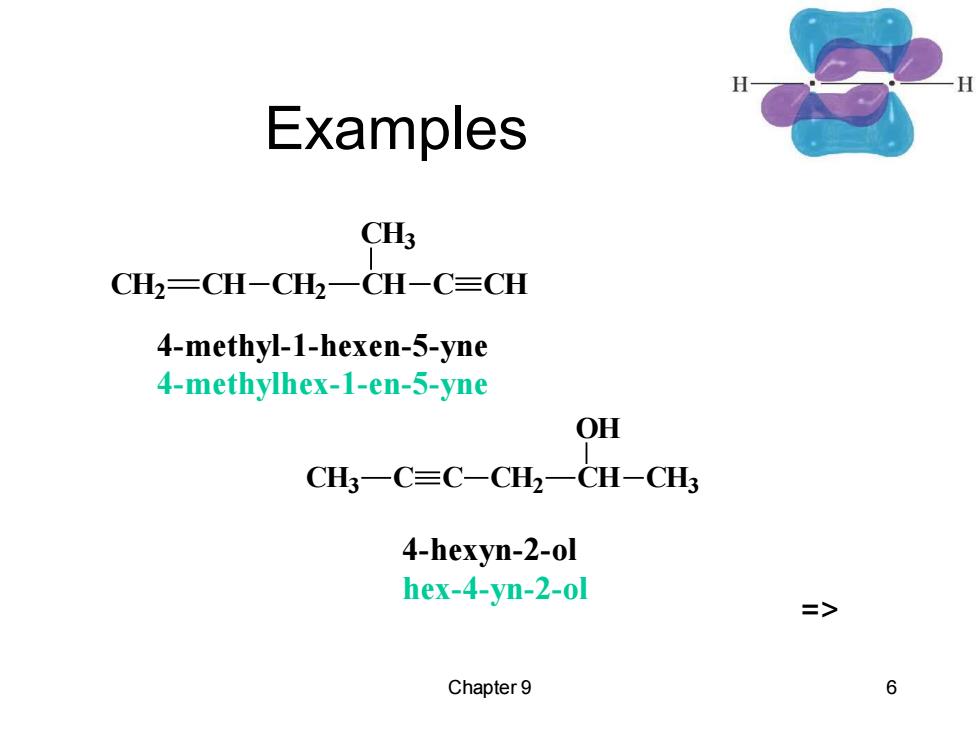
Examples C CH2=CH-CH2一CH-C=CH 4-methyl-1-hexen-5-yne 4-methylhex-1-en-5-yne OH CH3一C≡C-CH2一CH-CH3 4-hexyn-2-ol hex-4-yn-2-ol => Chapter 9 6
Chapter 9 6 Examples CH2 CH CH2 CH CH3 C CH 4-methyl-1-hexen-5-yne 4-methylhex-1-en-5-yne CH3 C C CH2 CH OH CH3 4-hexyn-2-ol hex-4-yn-2-ol =>
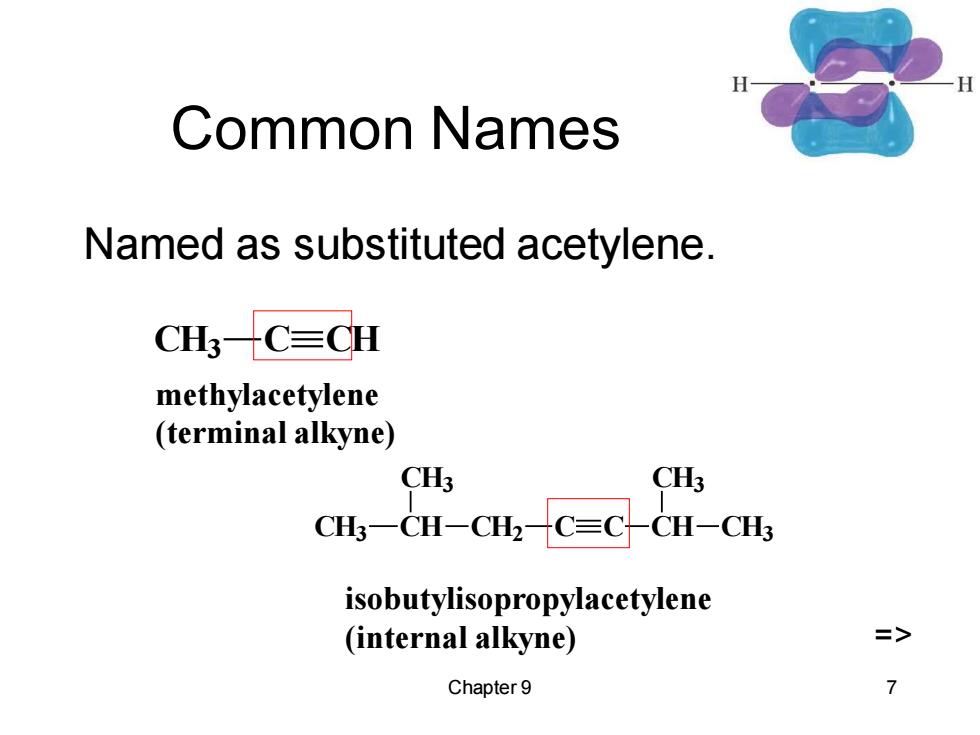
Common Names Named as substituted acetylene. CH3C≡CH methylacetylene (terminal alkyne) CH3 CH3 CH3一CH-CH2C=CCH-CH3 isobutylisopropylacetylene (internal alkyne) Chapter 9 7
Chapter 9 7 Common Names Named as substituted acetylene. CH3 C CH methylacetylene (terminal alkyne) CH3 CH CH3 CH2 C C CH CH3 CH3 isobutylisopropylacetylene (internal alkyne) =>
Physical Properties Nonpolar,insoluble in water. Soluble in most organic solvents. Boiling points similar to alkane of same size. Less dense than water. Up to 4 carbons,gas at room temperature. 二> Chapter 9 8
Chapter 9 8 Physical Properties • Nonpolar, insoluble in water. • Soluble in most organic solvents. • Boiling points similar to alkane of same size. • Less dense than water. • Up to 4 carbons, gas at room temperature. =>
Acetylene Acetylene is used in welding torches. In pure oxygen,temperature of flame reaches2800°C. It would violently decompose to its elements,but the cylinder on the torch contains crushed firebrick wet with acetone to moderate it. => Chapter 9 9
Chapter 9 9 Acetylene • Acetylene is used in welding torches. • In pure oxygen, temperature of flame reaches 2800C. • It would violently decompose to its elements, but the cylinder on the torch contains crushed firebrick wet with acetone to moderate it. =>
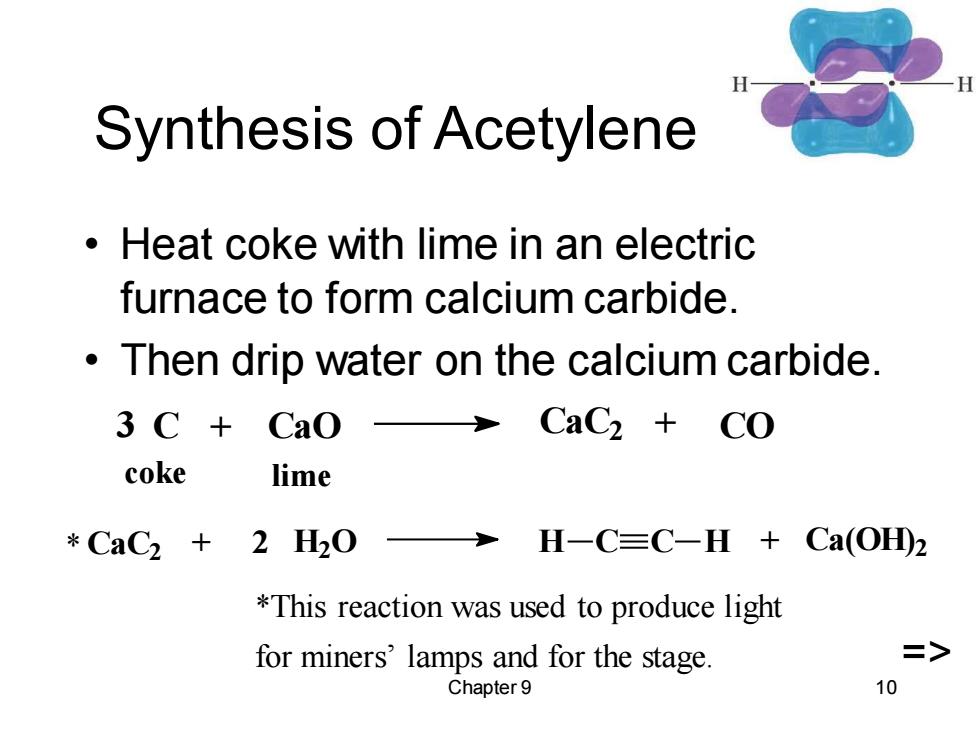
Synthesis of Acetylene Heat coke with lime in an electric furnace to form calcium carbide. Then drip water on the calcium carbide. 3 C+Cao>CaC2 CO coke lime *CaC2+2H20 H-C≡C-H+Ca(OHD2 *This reaction was used to produce light for miners'lamps and for the stage. => Chapter 9 10
Chapter 9 10 Synthesis of Acetylene • Heat coke with lime in an electric furnace to form calcium carbide. • Then drip water on the calcium carbide. CaC2 + 2 H2O H C C H + Ca(OH) 2 3 C + CaO CaC2 + CO coke lime *This reaction was used to produce light for miners’ lamps and for the stage. => *