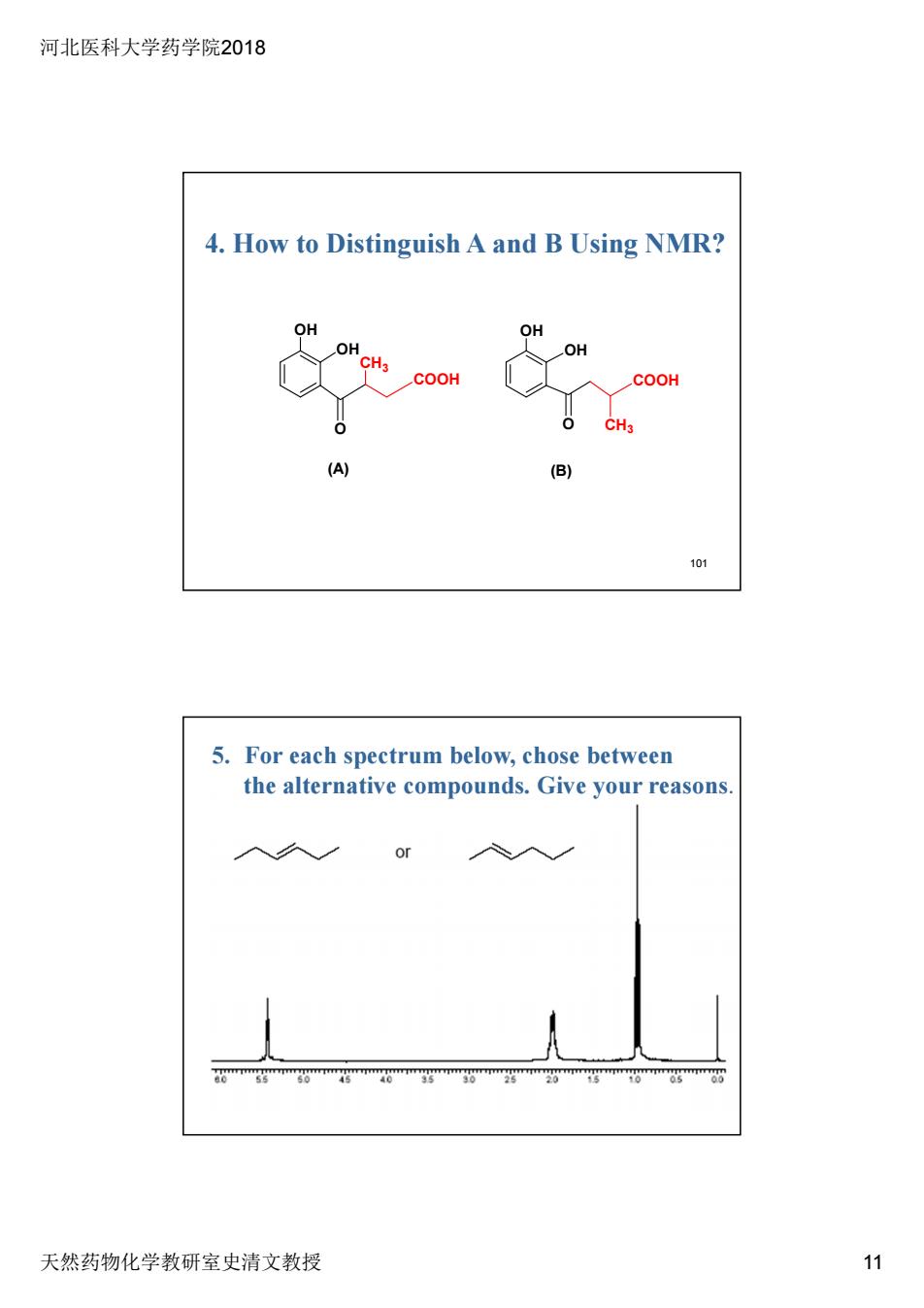
河北医科大学药学院2018 4.How to Distinguish A and B Using NMR? OH OH COOH COOH CH. 101 5.For each spectrum below,chose between the alternative compounds.Give your reasons 入久 天然药物化学教研室史清文教授 11
河北医科大学药学院2018 天然药物化学教研室史清文教授 11 101 4. How to Distinguish A and B Using NMR? OH OH OH OH O COOH CH3 COOH O CH3 (A) (B) 5. For each spectrum below, chose between the alternative compounds. Give your reasons
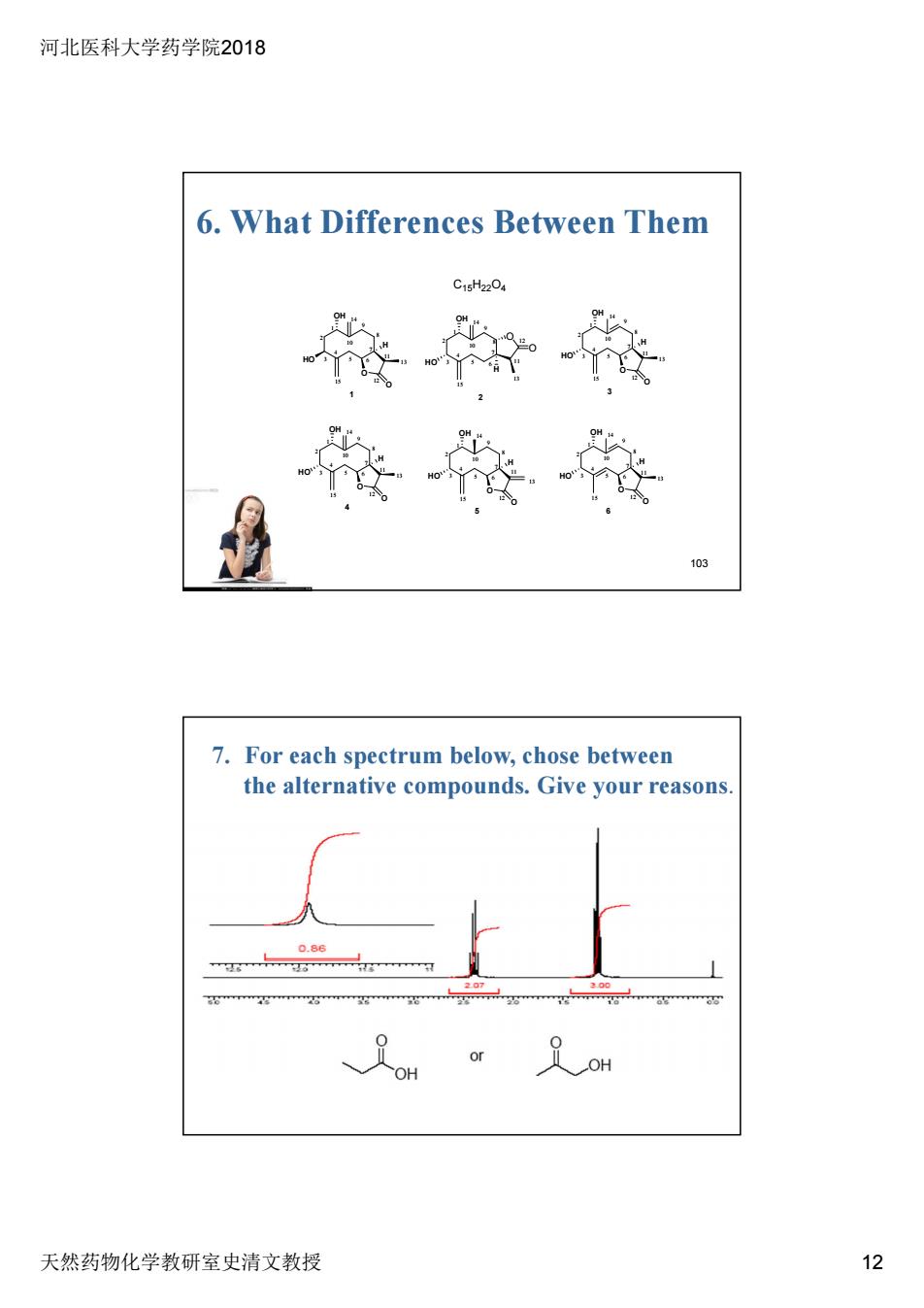
河北医科大学药学院2018 6.What Differences Between Them 15220 7.For each spectrum below,chose between the alternative compounds.Give your reasons 天然药物化学教研室史清文教授 12
河北医科大学药学院2018 天然药物化学教研室史清文教授 12 103 6. What Differences Between Them O OH HO O 1 4 7 10 13 15 2 3 5 6 8 9 11 12 14 1 H OH HO 2 4 7 10 13 15 2 3 5 6 8 9 11 12 14 1 H O O O OH HO O 4 4 7 10 13 15 2 3 5 6 8 9 11 12 14 1 H O OH HO O 5 4 7 10 13 15 2 3 5 6 8 9 11 12 14 1 H O OH HO O 3 4 7 10 13 15 2 3 5 6 8 9 11 12 14 1 H O OH HO O 6 4 7 10 13 15 2 3 5 6 8 9 11 12 14 1 H C15H22O4 7. For each spectrum below, chose between the alternative compounds. Give your reasons
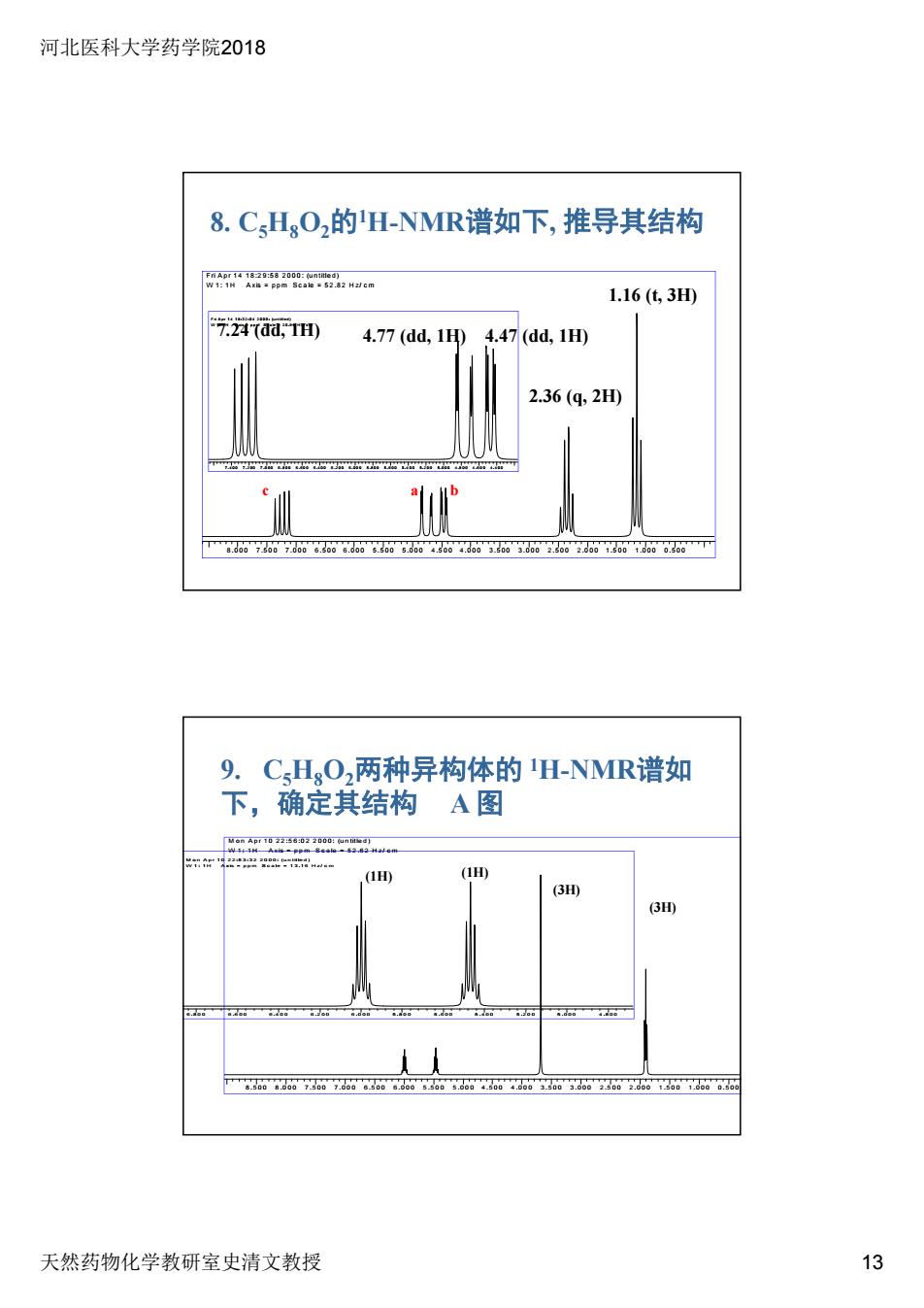
河北医科大学药学院2018 8.CHgO2的H-NMR谱如下,推导其结构 w":w 1.16(63H 4.77(dd,1D4.47(dd,1D 2.36(4,2H 9.CHO,两种异构体的H-NMR谱如 下,确定其结构A图 1H) 3 天然药物化学教研室史清文教授 13
河北医科大学药学院2018 天然药物化学教研室史清文教授 13 8. C5H8O2的1H-NMR谱如下, 推导其结构 Fri A p r 1 4 1 8 :2 9 :5 8 2 0 0 0 : (u n title d ) W 1 : 1 H A xis = p p m S c a le = 5 2 .8 2 H z/ c m 8 .0 0 0 7 .5 0 0 7 .0 0 0 6 .5 0 0 6 .0 0 0 5 .5 0 0 5 .0 0 0 4 .5 0 0 4 .0 0 0 3 .5 0 0 3 .0 0 0 2 .5 0 0 2 .0 0 0 1 .5 0 0 1 .0 0 0 0 .5 0 0 Fri A p r 1 4 1 8 :3 2 :0 4 2 0 0 0 : (u n title d ) W 1 : 1 H A xis = p p m S c a le = 2 0 .9 9 H z/ c m 7 .4 0 0 7 .2 0 0 7 .0 0 0 6 .8 0 0 6 .6 0 0 6 .4 0 0 6 .2 0 0 6 .0 0 0 5 .8 0 0 5 .6 0 0 5 .4 0 0 5 .2 0 0 5 .0 0 0 4 .8 0 0 4 .6 0 0 4 .4 0 0 7.24 (dd, 1H) 4.77 (dd, 1H) 4.47 (dd, 1H) 1.16 (t, 3H) 2.36 (q, 2H) c a b 9. C5H8O2两种异构体的 1H-NMR谱如 下,确定其结构 A 图 M o n A p r 1 0 2 2 :5 6 :0 2 2 0 0 0 : (u n title d ) W 1 : 1 H A xis = p p m S c a le = 5 2 .6 2 H z/ c m 8 .5 0 0 8 .0 0 0 7 .5 0 0 7 .0 0 0 6 .5 0 0 6 .0 0 0 5 .5 0 0 5 .0 0 0 4 .5 0 0 4 .0 0 0 3 .5 0 0 3 .0 0 0 2 .5 0 0 2 .0 0 0 1 .5 0 0 1 .0 0 0 0 .5 0 0 M o n A p r 1 0 2 2 :5 3 :3 2 2 0 0 0 : (u n title d ) W 1 : 1 H A xis = p p m S c a le = 1 3 .1 6 H z/ c m 6 .8 0 0 6 .6 0 0 6 .4 0 0 6 .2 0 0 6 .0 0 0 5 .8 0 0 5 .6 0 0 5 .4 0 0 5 .2 0 0 5 .0 0 0 4 .8 0 0 (3H) (3H) (1H) (1H)

河北医科大学药学院2018 B图 CsH3O2 wea a1.87d3H b3.71s3H c5.83m1 d7.03m1H 6。,。,。,a。 10.Assigin H,Hp,H.and Ha of Aspirin? 远程耦合常数 2 天然药物化学教研室史清文教授 14
河北医科大学药学院2018 天然药物化学教研室史清文教授 14 B 图 M o n A p r 1 0 2 3 :0 7 :0 6 2 0 0 0 : (u n title d ) W 1 : 1 H A xis = p p m S c a le = 7 6 .7 9 H z/ c m 1 0 .0 0 0 9 .0 0 0 8 .0 0 0 7 .0 0 0 6 .0 0 0 5 .0 0 0 4 .0 0 0 3 .0 0 0 2 .0 0 0 1 .0 0 0 0 .0 0 0 -1 .0 0 0 -2 .0 0 0 a 1.87 d 3H b 3.71 s 3H c 5.83 m 1H d 7.03 m 1H C5H8O2 10. Assigin Ha, Hb, Hc and Hd of Aspirin? td td dd dd 远程耦合常数 C O 1 2 3 4 O OH CH3 O

河北医科大学药学院2018 H-NMR of Salicylic Acid H118 11.图谱解析-信号归属 聘转 MW-180 J=15.9Hz J=16.2Hz 天然药物化学教研室史清文教授 15
河北医科大学药学院2018 天然药物化学教研室史清文教授 15 1H-NMR of Salicylic Acid 11. 图谱解析-信号归属 MW = 180 HO OH OH J=15.9 Hz O J=16.2 Hz