Sec 2 Hybridization 0球4大号 Molecular Shapes Hybridized orbitals are lower in energy because electron pairs are farther apart. Hybridization is LCAO within one atom, just prior to bonding. linear combination of atomic orbitals---LCAO
Sec 2 Hybridization & Molecular Shapes Hybridized orbitals are lower in energy because electron pairs are farther apart. Hybridization is LCAO within one atom, just prior to bondin g. linear combination of atomic orbitals--- LCAO

0际人号 Theories Valence Bond Theory价键理论 concerns itself with the formation of sigma and pi bonds. VSEPR:Valence Shell Electron Pair Repulsion Theory .The premise of VSEPR is that the valence electron pairs surrounding an atom mutually repel each other,and will therefore adopt an arrangement that minimizes this repulsion, thus determining the molecular geometry. OVSEPR addresses molecular shape through orbitals that are energetically accessible for bonding. Molecular Orbital Theory model for understanding how atoms and electrons are assembled into molecules and polyatomic ions. ■Hybridization Theory杂化轨道理论 a covalent bond is formed by the overlap of two singly occupied hybrid or atomic orbitals. .Hybrid atomic orbitals are created by mixing together atomic orbitals to form an equal number of new hybrid atomic orbitals
Theories Valence Bond Theory价键理论 zconcerns itself with the formation of sigma and pi bonds. VSEPR: Valence Shell Electron Pair Repulsion Theory zThe premise of VSEPR is that the valence electron pairs surrounding an atom mutually repel each other, and will therefore adopt an arrangement that minimizes this repulsion, thus determining the molecular g eome try. zVSEPR add resses molecular shape through orbitals that are energetically accessible for bonding. Molecular Orbital Theory zmodel for understanding how atoms and electrons are assembled into molecules and p olyatomic ions. Hybridization Theory杂化轨道理论 za cov alent bond is formed by the ove rlap o f two singly occupied hybrid o r atomic orbitals. zHybrid ato mic orbitals are created by mixing tog ether ato mic orbitals to form an equal number of new hybrid atomic orbitals
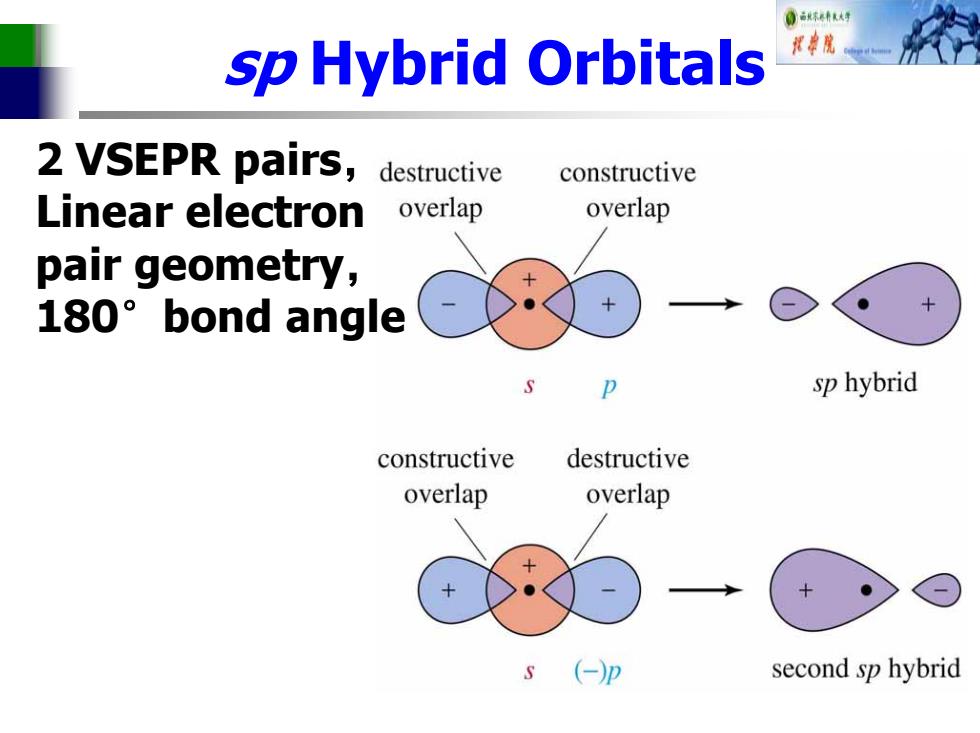
0古林大号 sp Hybrid Orbitals 2 VSEPR pairs,destructive constructive Linear electron overlap overlap pair geometry, 180°bond angle sp hybrid constructive destructive overlap overlap (-p second sp hybrid
sp Hybrid Orbitals 2 VSEPR pairs, Linear electron pair geometry, 180°bond angle

0球有4 sp2 Hybrid Orbitals 院 3 VSEPR pairs,Trigonal planar e-pair geometry,120 bond angle sp2hybrid orbitals unhybridized P:orbital 120 、120° three sp2hybrid orbitals superimposed sp2hybrid carbon atom (viewed from the side) Copyright 2005 Pearson Prentice Hall,Inc
sp2 Hybrid Orbitals 3 VSEPR pairs, Trigonal planar e- pair geometry, 120° bond angle

0 样串院 120 SD2 sp2 Sp2 side view top view AdGif-UNREGISTERED To form a planer carbon
To form a planer carbon