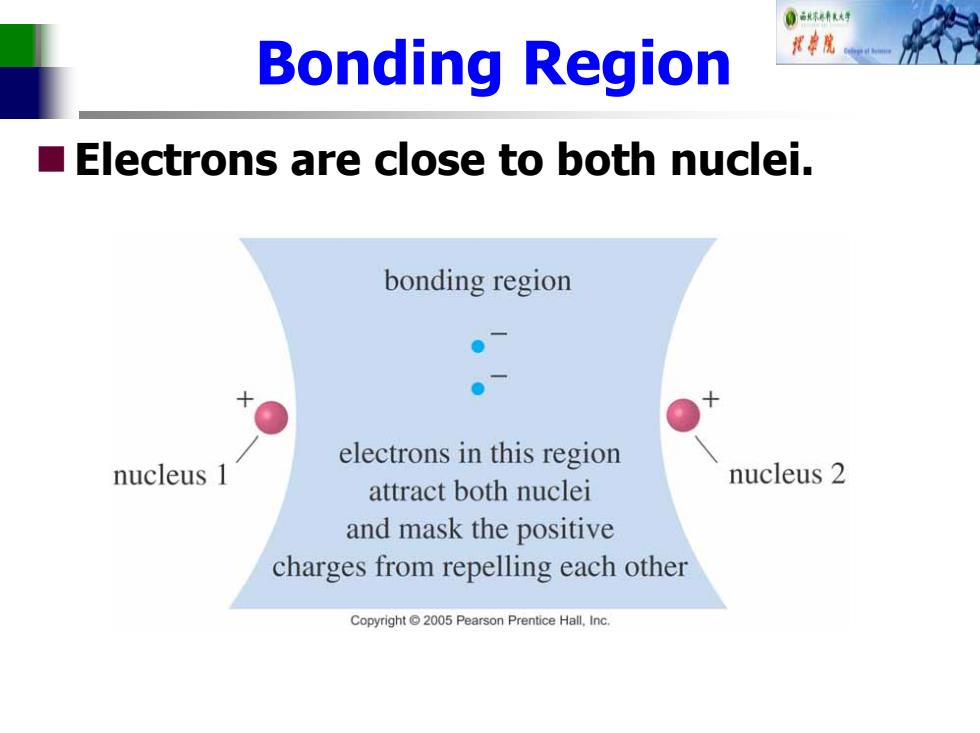
0林有 Bonding Region ■ Electrons are close to both nuclei. bonding region ●一 electrons in this region nucleus 1 nucleus 2 attract both nuclei and mask the positive charges from repelling each other Copyright 2005 Pearson Prentice Hall,Inc
Bonding Region Electrons are close to both nuclei
0木有人写 Sigma(o)Bonding Electron density lies between the nuclei. A bond may be formed by s-s,p-p,s-p, or hybridized orbital overlaps. The bonding MO is lower in energy than the original atomic orbitals. The antibonding MO is higher in energy than the atomic orbitals
Sigma(σ) Bonding Electron density lies between the nuclei. A bond may be formed by s-s, p-p, s-p, or hybridized orbital overlaps. The bonding MO is lower in energy than the original atomic orbitals. The antibonding MO is higher in energy than the atomic orbitals

0林司 Bonding Molecular Orbital Two hydrogens,1s constructive overlap Constructive Interaction:The two 1s orbitals are in phase and have the same sign. add bonding molecular orbital represented by: o-bonding MO Copyright2005 Pearson Prentice Hall,Inc
Bonding Molecular Orbital Two hydrogens, 1s constructive overlap

0林人号 Anti-Bonding Molecular Orbital Two hydrogens,destructive overlap. Destructive interaction:The two Is orbitals are out of phase. add antibonding molecular orbital represented by: node Copyright2005 Pearson Prentice Hall,Inc
Anti-Bonding Molecular Orbital Two hydrogens, destructive overlap
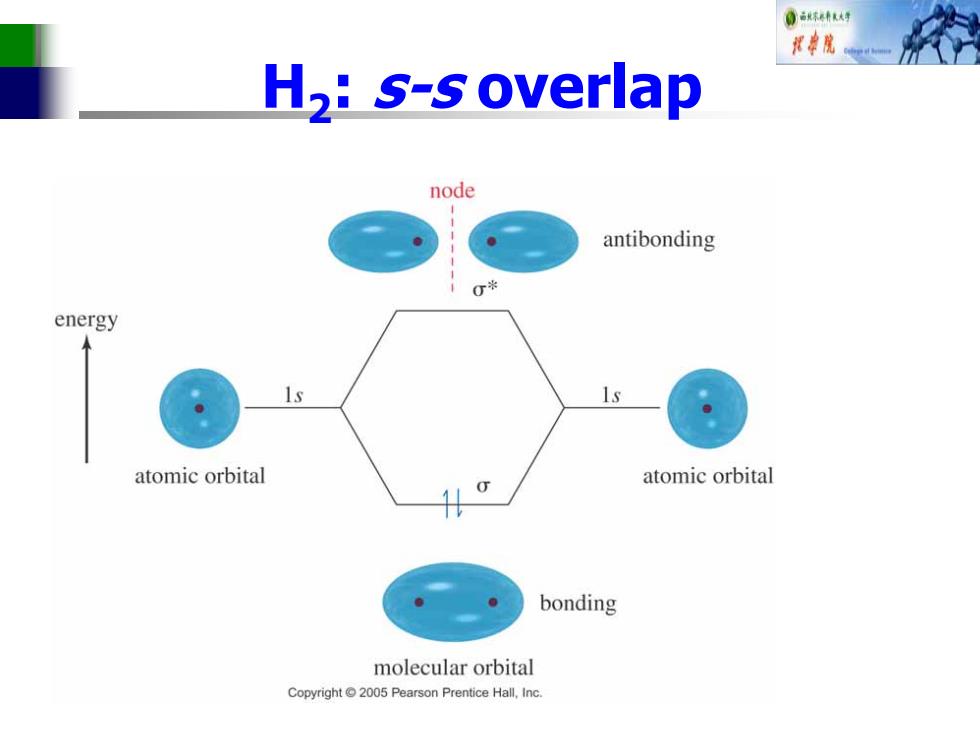
0 样串院 H,:s-s overlap node antibonding 0兴 energy atomic orbital atomic orbital bonding molecular orbital Copyright2005 Pearson Prentice Hall,Inc
H2: s-s overlap