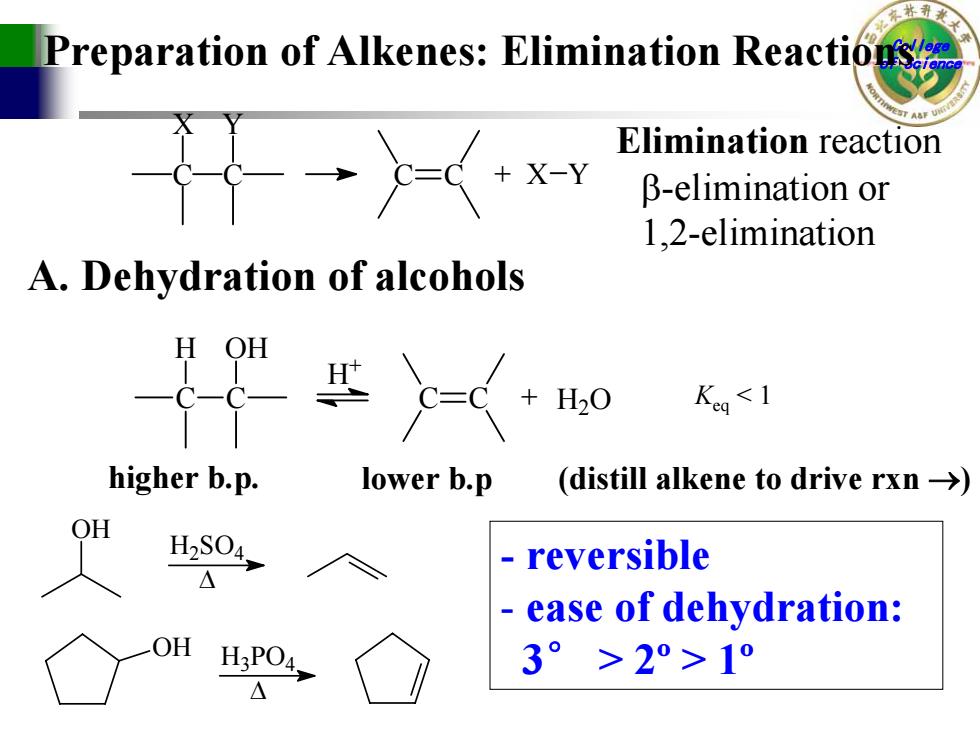
Preparation of Alkenes:Elimination Reactions Elimination reaction X-Y B-elimination or 1.2-elimination A.Dehydration of alcohols H OH H20 Keg<1 higher b.p. lower b.p (distill alkene to drive rxn -> H2S04, reversible ease of dehydration: H3PO4 3°>2°>1°
College Preparation of Alkenes: Elimination Reactionsof Science C X C Y C C + X Y Elimination reaction β-elimination or 1,2-elimination A. Dehydration of alcohols C H C OH C C + H 2 O H + Keq < 1 higher b.p. lower b.p (distill alkene to drive rxn → ) OH H 2SO 4 ∆ OH H 3PO 4 ∆ - r e v e r s i b l e - ease of dehydration: 3 ° > 2º > 1º
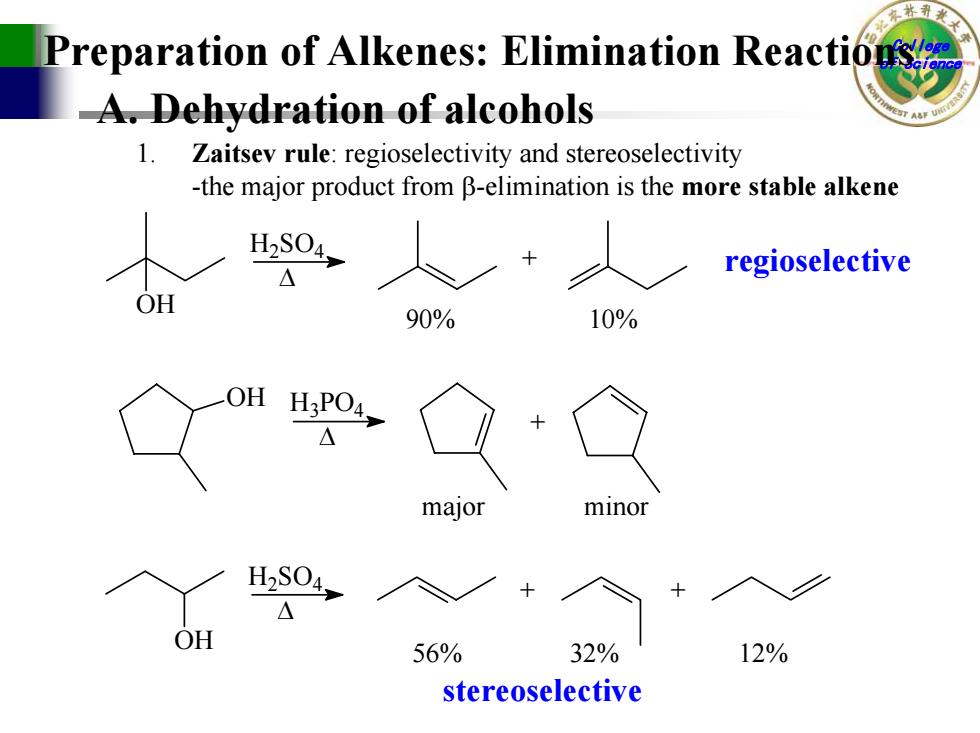
Preparation of Alkenes:Elimination Reaction A.Dehydration of alcohols 1.Zaitsev rule:regioselectivity and stereoselectivity -the major product from B-elimination is the more stable alkene 人 s0人 regioselective O 90% 10% OH HaPO4 major minor H2S04, △ OH 56% 32% 12% stereoselective
College of Science A. Dehydration of alcohols Preparation of Alkenes: Elimination Reactions 1. Zaitsev rule: regioselectivity and stereoselectivity -the major product from β-elimination is the more stable alken e OH + H 2SO 4 ∆ 90% 10% OH H 3PO 4 ∆ + OH + + H 2SO 4 ∆ 56% 32% 12% major minor regioselective stereoselective
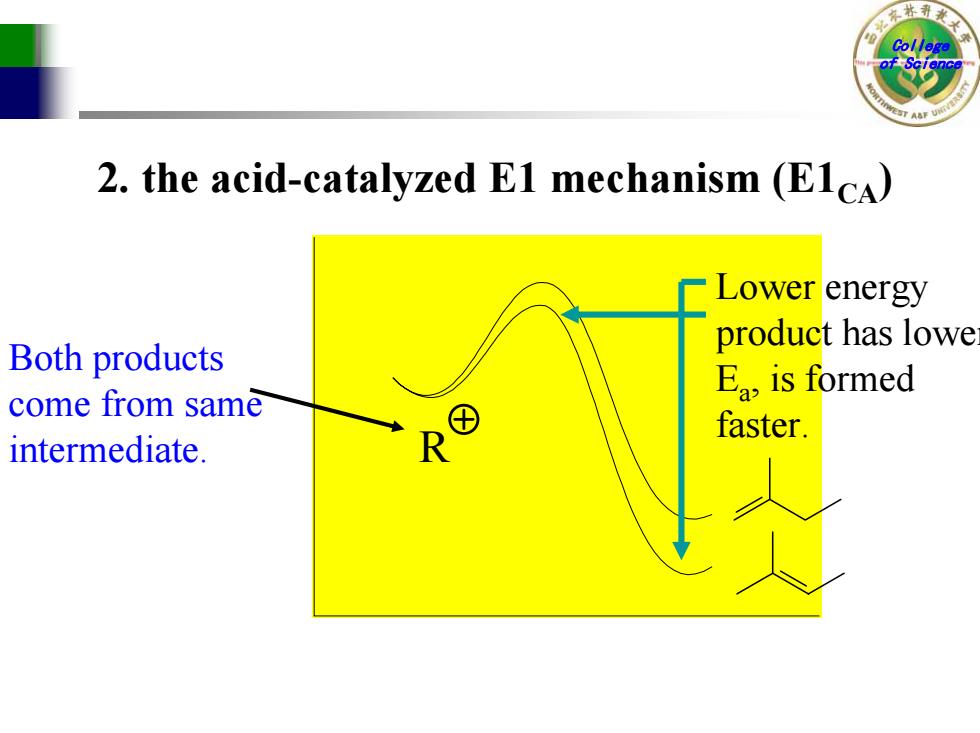
Colle 2.the acid-catalyzed E1 mechanism (EICA) Lower energy product has lowe Both products Ea is formed come from same faster. intermediate
College of Science 2. the acid-catalyzed E1 mechanism (E1CA ) R Both products come from same intermediate. Lower energy product has lowe r E a, is formed faster
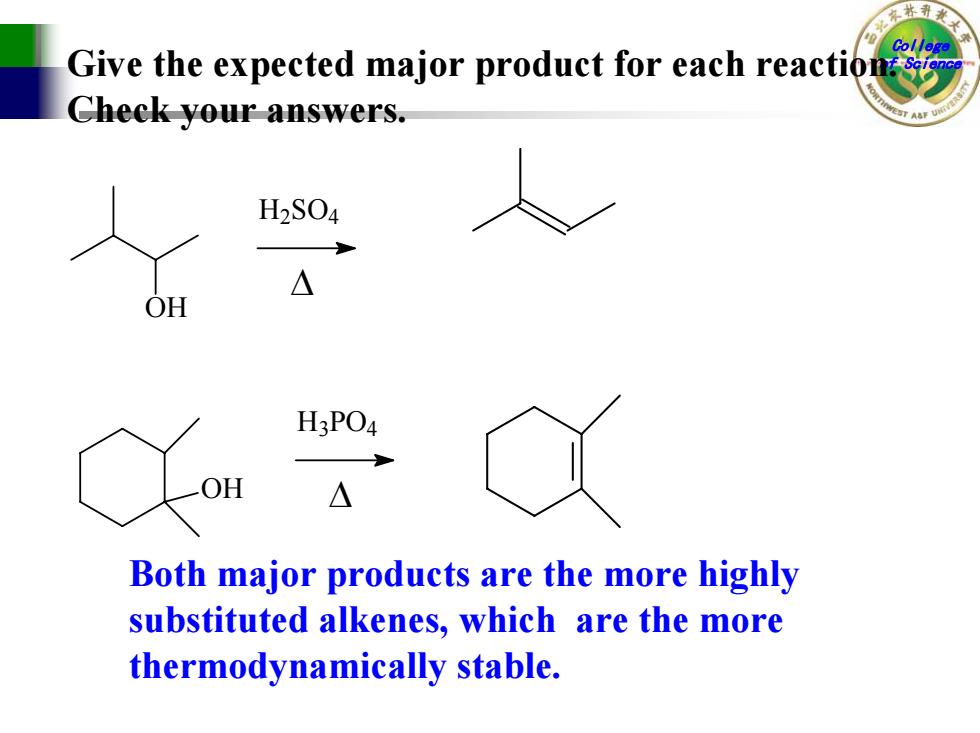
林 Give the expected major product for each reactions Check your answers. H2S04 △ OH H3PO4 OH △ Both major products are the more highly substituted alkenes,which are the more thermodynamically stable
College Give the expected major product for each reaction.of Science Check your answers. OH H 2SO 4 ∆ OH H 3PO 4 ∆ Both major products are the more highly substituted alkenes, which are the more thermodynamically stable
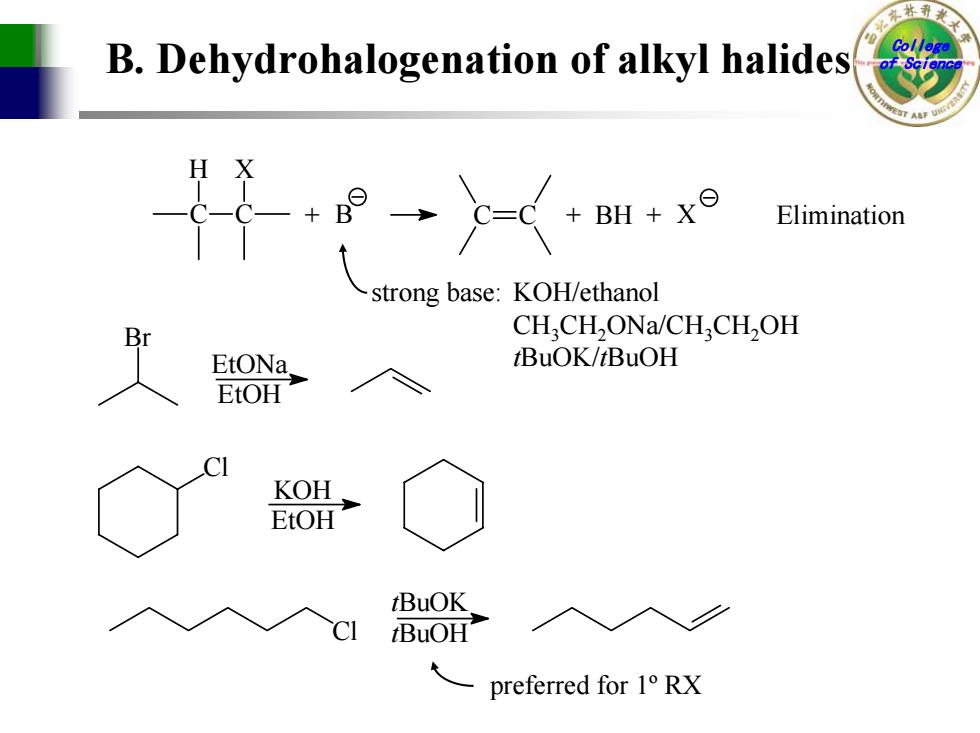
:Q B.Dehydrohalogenation of alkyl halides Colle BH+x Elimination strong base:KOH/ethanol 8, CHCH,ONa/CH,CH,OH EtONa, tBuOK/tBuOH EtOH KOH EtOH tBuOK. BuOH preferred for 1 RX
College B. Dehydrohalogenation of alkyl halides of Science C H C X + B C C + BH + X strong base: K O H/ethanol CH 3CH 2ONa/CH 3CH 2OH tBuOK/ tBuOH Br E tONa Et O H Cl KO H Et O H Cl tBu O K tBu O H Elimination preferred for 1º RX