
版权所有:华东理工大学物理化学教研室 6 2) Exergonic and endergonic reactions 9.1 The Gibbs energy minimum The spontaneity of a reaction at constant temperature and pressure is expressed by the reaction Gibbs energy: If ΔrG <0, the forward reaction is spontaneous; it is called exergonic (work-producing) If ΔrG >0, the reverse reaction is spontaneous; it is called endergonic (work-consuming) If ΔrG = 0, the reaction is at equilibrium; it is spontaneous in neither direction, and neither exergonic nor endergonic
版权所有:华东理工大学物理化学教研室 6 2) Exergonic and endergonic reactions 9.1 The Gibbs energy minimum The spontaneity of a reaction at constant temperature and pressure is expressed by the reaction Gibbs energy: If ΔrG <0, the forward reaction is spontaneous; it is called exergonic (work-producing) If ΔrG >0, the reverse reaction is spontaneous; it is called endergonic (work-consuming) If ΔrG = 0, the reaction is at equilibrium; it is spontaneous in neither direction, and neither exergonic nor endergonic
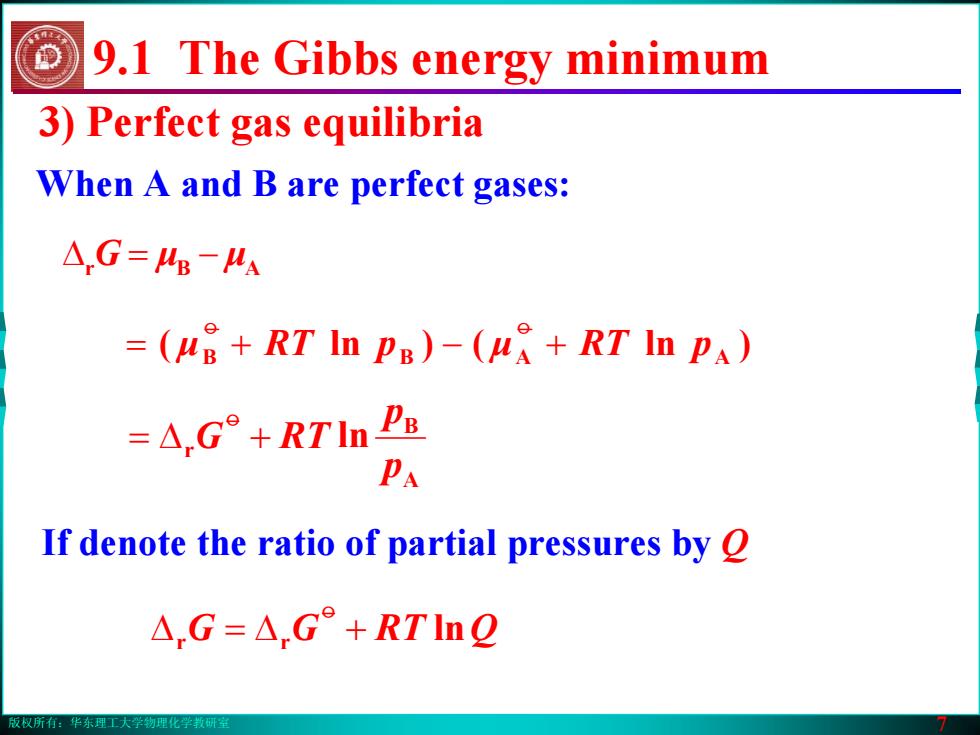
版权所有:华东理工大学物理化学教研室 7 3) Perfect gas equilibria When A and B are perfect gases: If denote the ratio of partial pressures by Q Δ G = − μμ ABr 9.1 The Gibbs energy minimum ( μB += pRT B ()ln μ A +− pRT A )ln o o A B r ln p p +Δ= RTGo r r +Δ=Δ lnQRTGG o
版权所有:华东理工大学物理化学教研室 7 3) Perfect gas equilibria When A and B are perfect gases: If denote the ratio of partial pressures by Q Δ G = − μμ ABr 9.1 The Gibbs energy minimum ( μB += pRT B ()ln μ A +− pRT A )ln o o A B r ln p p +Δ= RTGo r r +Δ=Δ lnQRTGG o
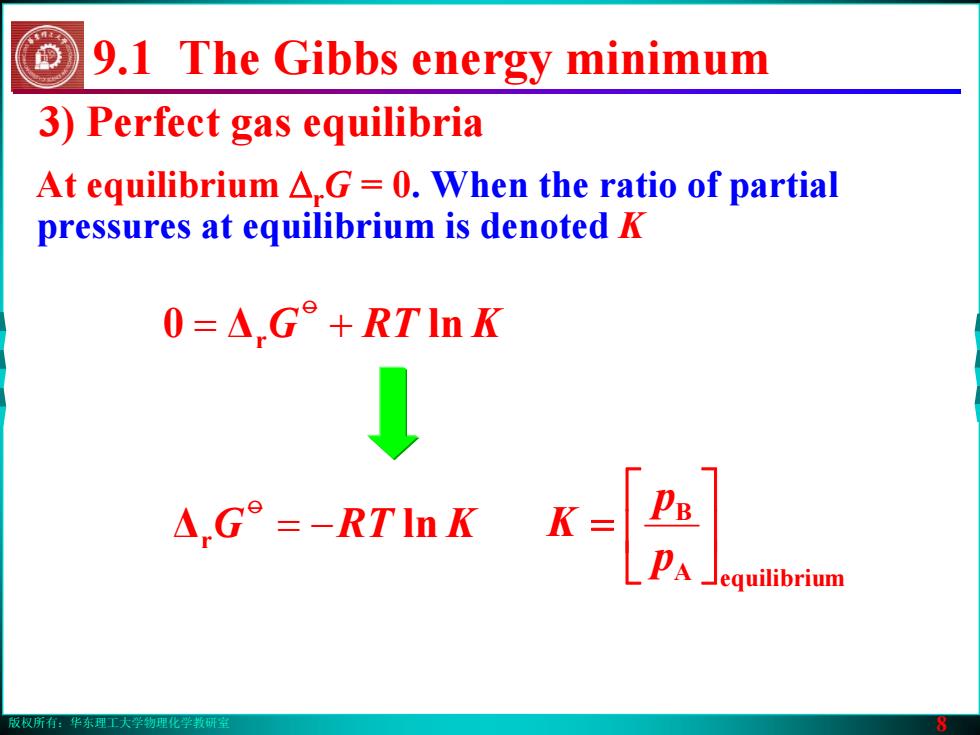
版权所有:华东理工大学物理化学教研室 8 3) Perfect gas equilibria At equilibrium ΔrG = 0. When the ratio of partial pressures at equilibrium is denoted K 9.1 The Gibbs energy minimum Δr −= ln KRTGo 0 Δr += ln KRTGo A equilibrium B ⎥⎦⎤ ⎢⎣⎡ = pp K
版权所有:华东理工大学物理化学教研室 8 3) Perfect gas equilibria At equilibrium ΔrG = 0. When the ratio of partial pressures at equilibrium is denoted K 9.1 The Gibbs energy minimum Δr −= ln KRTGo 0 Δr += ln KRTGo A equilibrium B ⎥⎦⎤ ⎢⎣⎡ = pp K

版权所有:华东理工大学物理化学教研室 9 3) Perfect gas equilibria 9.1 The Gibbs energy minimum When > 0, K< 1: at equilibrium the partial pressure of A exceeds that of B, the reactant A is favoured in the equilibrium. o ΔrG When < 0, K > 1 : at equilibrium the partial pressure of B exceeds that of A, the product B is favoured in the equilibrium. o ΔrG
版权所有:华东理工大学物理化学教研室 9 3) Perfect gas equilibria 9.1 The Gibbs energy minimum When > 0, K< 1: at equilibrium the partial pressure of A exceeds that of B, the reactant A is favoured in the equilibrium. o ΔrG When < 0, K > 1 : at equilibrium the partial pressure of B exceeds that of A, the product B is favoured in the equilibrium. o ΔrG
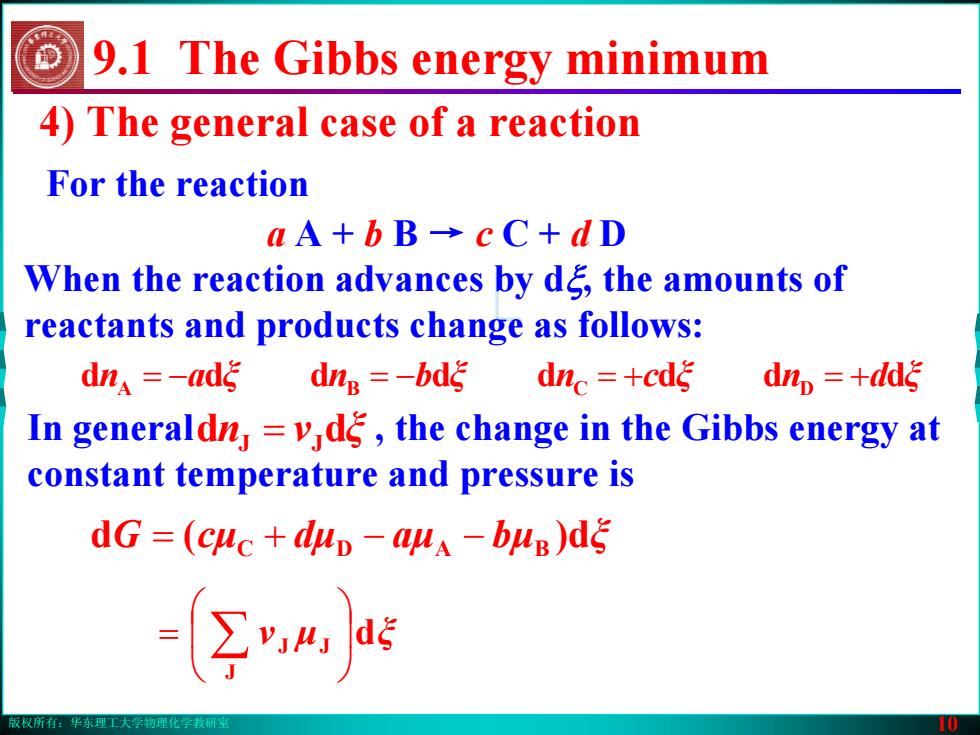
版权所有:华东理工大学物理化学教研室 10 4) The general case of a reaction For the reaction dξμν J JJ ⎟⎠⎞ ⎜⎝⎛ = ∑ a A + b B → c C + d D When the reaction advances by dξ, the amounts of reactants and products change as follows: an ξ bn ξ cn ξ dn dddddddd ξ A = − B = − C = + D = + In general , the change in the Gibbs energy at constant temperature and pressure is n dd ξν = JJ G (d cμ dμ aμ bμ d) ξ = C + D − − BA 9.1 The Gibbs energy minimum
版权所有:华东理工大学物理化学教研室 10 4) The general case of a reaction For the reaction dξμν J JJ ⎟⎠⎞ ⎜⎝⎛ = ∑ a A + b B → c C + d D When the reaction advances by dξ, the amounts of reactants and products change as follows: an ξ bn ξ cn ξ dn dddddddd ξ A = − B = − C = + D = + In general , the change in the Gibbs energy at constant temperature and pressure is n dd ξν = JJ G (d cμ dμ aμ bμ d) ξ = C + D − − BA 9.1 The Gibbs energy minimum