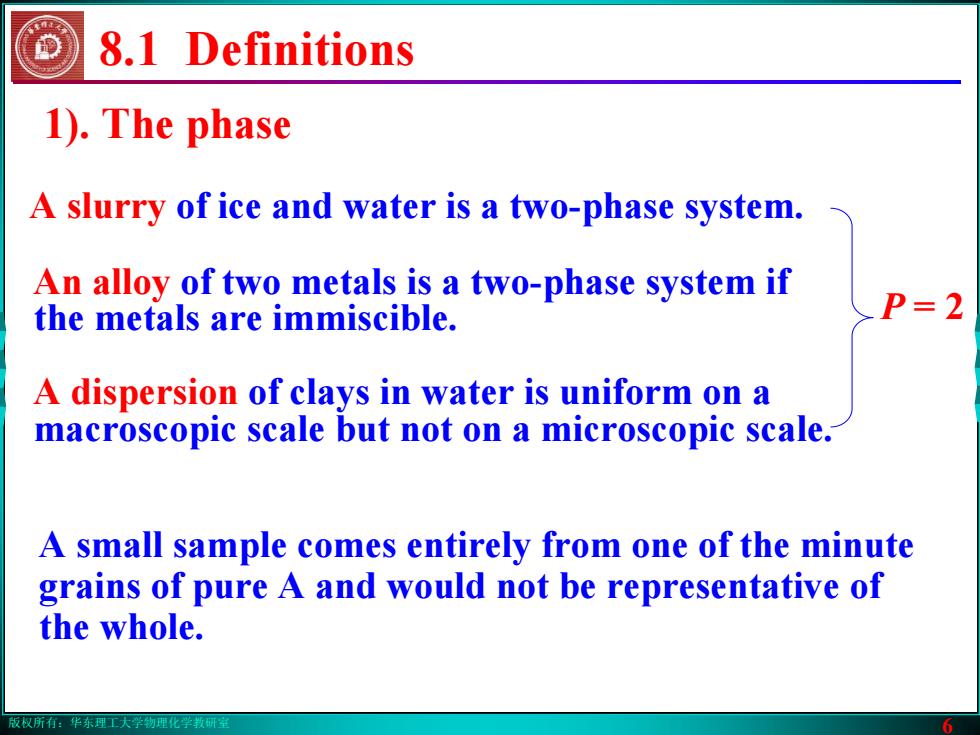
版权所有:华东理工大学物理化学教研室 6 8.1 Definitions 1). The phase An alloy of two metals is a two-phase system if the metals are immiscible. A slurry of ice and water is a two-phase system. P = 2 A dispersion of clays in water is uniform on a macroscopic scale but not on a microscopic scale. A small sample comes entirely from one of the minute grains of pure A and would not be representative of the whole
版权所有:华东理工大学物理化学教研室 6 8.1 Definitions 1). The phase An alloy of two metals is a two-phase system if the metals are immiscible. A slurry of ice and water is a two-phase system. P = 2 A dispersion of clays in water is uniform on a macroscopic scale but not on a microscopic scale. A small sample comes entirely from one of the minute grains of pure A and would not be representative of the whole
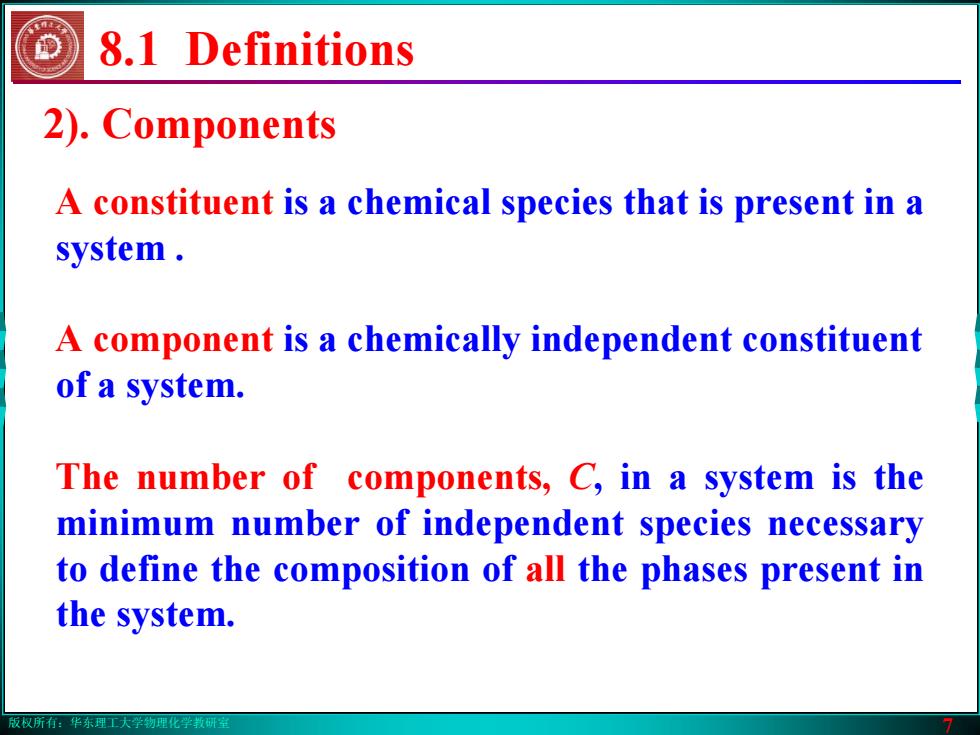
版权所有:华东理工大学物理化学教研室 7 8.1 Definitions 2). Components A constituent is a chemical species that is present in a system . A component is a chemically independent constituent of a system. The number of components, C, in a system is the minimum number of independent species necessary to define the composition of all the phases present in the system
版权所有:华东理工大学物理化学教研室 7 8.1 Definitions 2). Components A constituent is a chemical species that is present in a system . A component is a chemically independent constituent of a system. The number of components, C, in a system is the minimum number of independent species necessary to define the composition of all the phases present in the system

版权所有:华东理工大学物理化学教研室 8 8.1 Definitions 2). The number of components, C No chemical reactions The pure water is a one-component system, C = 1, because we need only the species H2O to specify its composition. Similarly, a mixture of ethanol and water is a two-component system, C = 2: we need the species H2O and C2H5OH to specify its composition. When no reaction takes place, the number of components is equal to the number of constituents
版权所有:华东理工大学物理化学教研室 8 8.1 Definitions 2). The number of components, C No chemical reactions The pure water is a one-component system, C = 1, because we need only the species H2O to specify its composition. Similarly, a mixture of ethanol and water is a two-component system, C = 2: we need the species H2O and C2H5OH to specify its composition. When no reaction takes place, the number of components is equal to the number of constituents
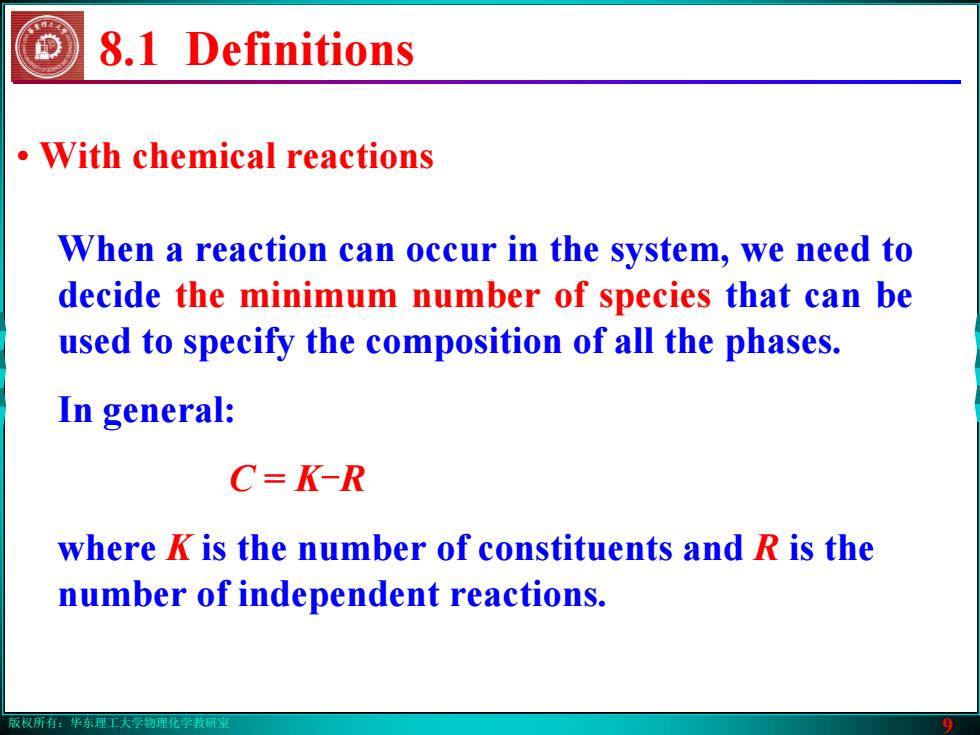
版权所有:华东理工大学物理化学教研室 9 8.1 Definitions • With chemical reactions When a reaction can occur in the system, we need to decide the minimum number of species that can be used to specify the composition of all the phases. In general: C = K-R where K is the number of constituents and R is the number of independent reactions
版权所有:华东理工大学物理化学教研室 9 8.1 Definitions • With chemical reactions When a reaction can occur in the system, we need to decide the minimum number of species that can be used to specify the composition of all the phases. In general: C = K-R where K is the number of constituents and R is the number of independent reactions
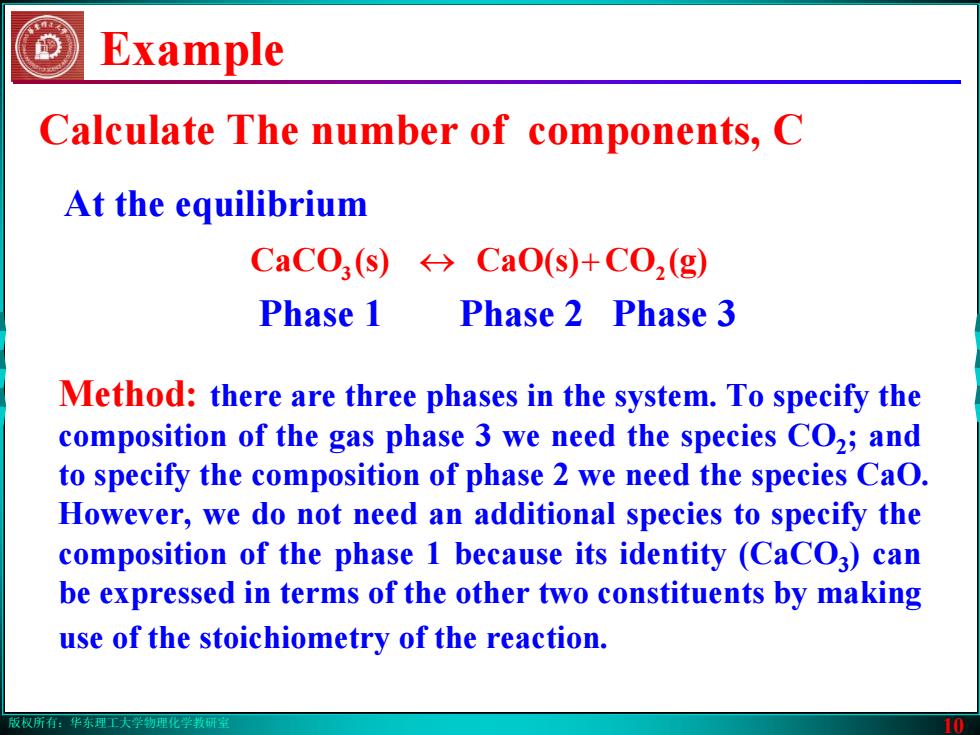
版权所有:华东理工大学物理化学教研室 10 Example Method: there are three phases in the system. To specify the composition of the gas phase 3 we need the species CO2; and to specify the composition of phase 2 we need the species CaO. However, we do not need an additional species to specify the composition of the phase 1 because its identity (CaCO3) can be expressed in terms of the other two constituents by making use of the stoichiometry of the reaction. At the equilibrium 3 ↔ CaO(s) (s)CaCO + 2 (g)CO Phase 1 Phase 2 Phase 3 Calculate The number of components, C
版权所有:华东理工大学物理化学教研室 10 Example Method: there are three phases in the system. To specify the composition of the gas phase 3 we need the species CO2; and to specify the composition of phase 2 we need the species CaO. However, we do not need an additional species to specify the composition of the phase 1 because its identity (CaCO3) can be expressed in terms of the other two constituents by making use of the stoichiometry of the reaction. At the equilibrium 3 ↔ CaO(s) (s)CaCO + 2 (g)CO Phase 1 Phase 2 Phase 3 Calculate The number of components, C