河北医科大学药学院2018 Ref:CDCB 7.26 pp m for 'H-NMR,77.16 ppm for C-NMIR eiesaaB1153e 股 盛和管室 德塑世色 安家 天然药物化学教研室史清文教授 6
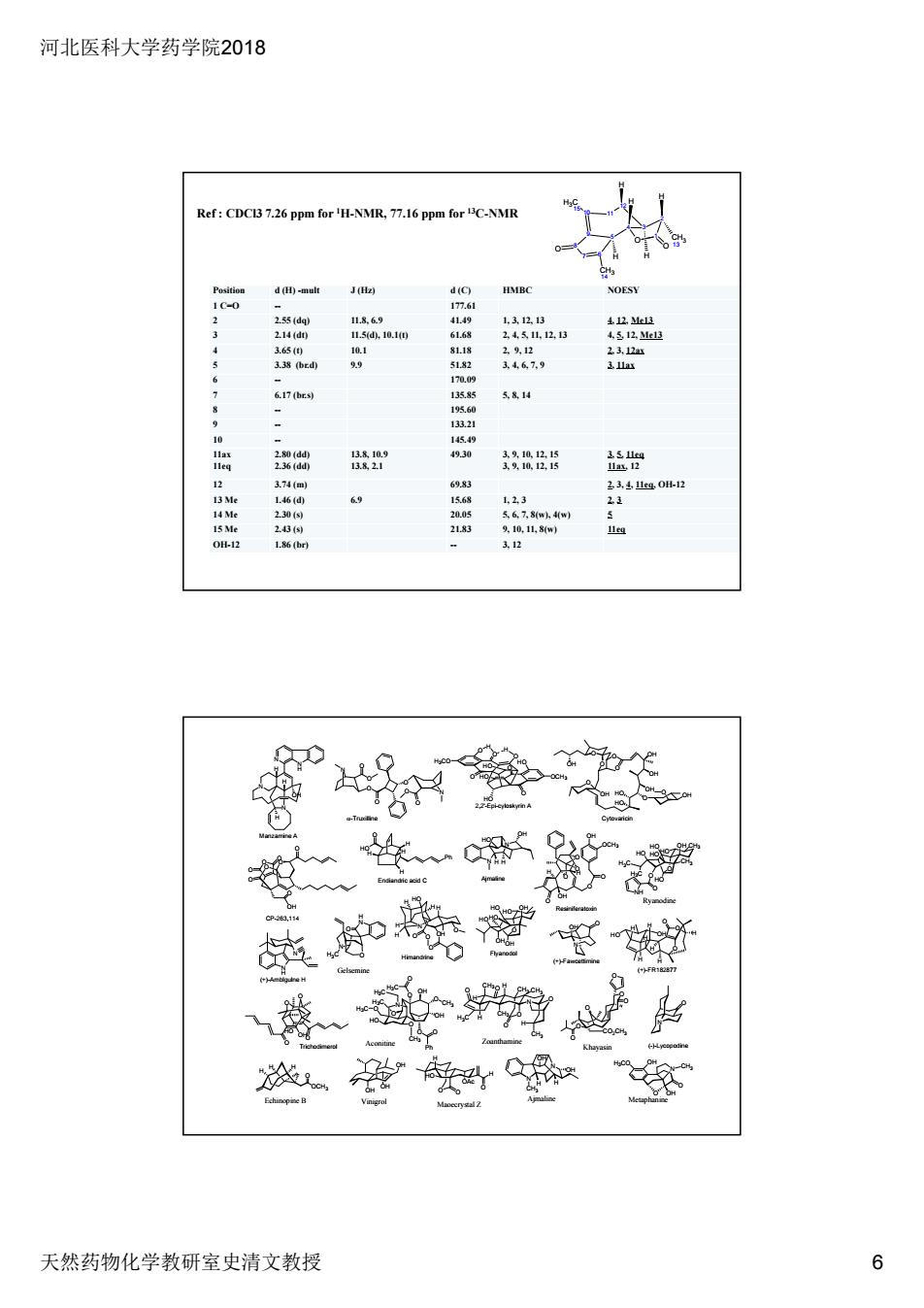
河北医科大学药学院2018 天然药物化学教研室史清文教授 6 Position d (H) -mult J (Hz) d (C) HMBC NOESY 1 C=O -- 177.61 2 2.55 (dq) 11.8, 6.9 41.49 1, 3, 12, 13 4, 12, Me13 3 2.14 (dt) 11.5(d), 10.1(t) 61.68 2, 4, 5, 11, 12, 13 4, 5, 12, Me13 4 3.65 (t) 10.1 81.18 2, 9, 12 2, 3, 12ax 5 3.38 (br.d) 9.9 51.82 3, 4, 6, 7, 9 3, 11ax 6 -- 170.09 7 6.17 (br.s) 135.85 5, 8, 14 8 -- 195.60 9 -- 133.21 10 -- 145.49 11ax 11eq 2.80 (dd) 2.36 (dd) 13.8, 10.9 13.8, 2.1 49.30 3, 9, 10, 12, 15 3, 9, 10, 12, 15 3, 5, 11eq 11ax, 12 12 3.74 (m) 69.83 2, 3, 4, 11eq, OH-12 13 Me 1.46 (d) 6.9 15.68 1, 2, 3 2, 3 14 Me 2.30 (s) 20.05 5, 6, 7, 8(w), 4(w) 5 15 Me 2.43 (s) 21.83 9, 10, 11, 8(w) 11eq OH-12 1.86 (br) -- 3, 12 Ref : CDCl3 7.26 ppm for 1H-NMR, 77.16 ppm for 13C-NMR 3 2 4 1 O O CH3 13 12 11 5 9 10 6 7 8 H3C 15 O CH3 14 H H H H H Manzamine A N N H H H H H Ph O HO Endiandric acid C 2,2'-Epi-cyloskyrin A Cytovaricin O O HO O O HO O OH OH OH O OH O OH OH (+)-Amblgulne H N H N (+)-FR182877 CP-263,114 O O O O O O O OH O -Truxilline O O O N O O N O O O H O O H O HO HO O OCH3 O HO H3CO HO Flyanodol O OH HO HO HO HO OH OH Ajmaline N N OH H H HO Himandrine (+)-Fawcettimine N O OH (-)-Lycopodine N O Resiniferatoxin O O OH O O O O H OH H N OH N H H H HO OH O O O H H H H H H H O O O O N HO H H O H H H H OCH3 HO OH O O O O Trichodimerol N OH O CH3 OH O Ph O HO H3C O O H3C O H3C O H3C O CH3 Aconitine N N OH OH H H CH3 Ajmaline N O H3CO OH CH3 Ryanodine H3C H3C O O O NH HO HO HO OH HO HO CH3 CH3 OCH3 O H H H Echinopine B O O H O OAc HO H Maoecrystal Z O O O O O CO2CH3 O Khayasin Metaphanine O OH N H N O O H3C Gelsemine OH OH OH Vinigrol Zoanthamine N O O O O O CH3 H3C CH3 CH3 CH3 CH3 H H H H
河北医科大学药学院2018 天麻素 积并用 天然药物化学教研室史清文教授 7
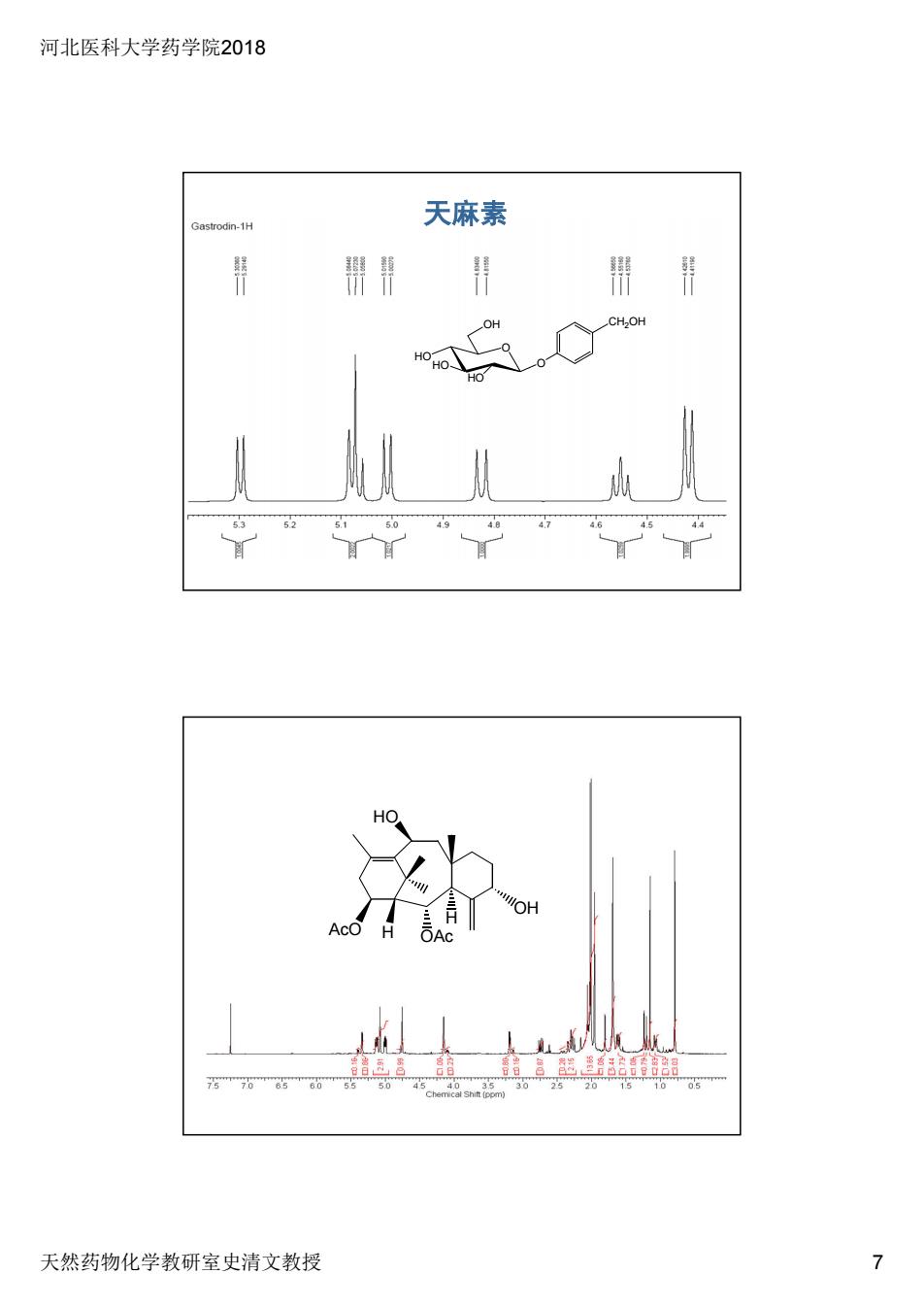
河北医科大学药学院2018 天然药物化学教研室史清文教授 7 CH2OH O O OH HO HOHO 天麻素 H OAc H OH HO AcO
河北医科大学药学院2018 7065605550454035302520151005 肉用周 天然药物化学教研室史清文教授 8
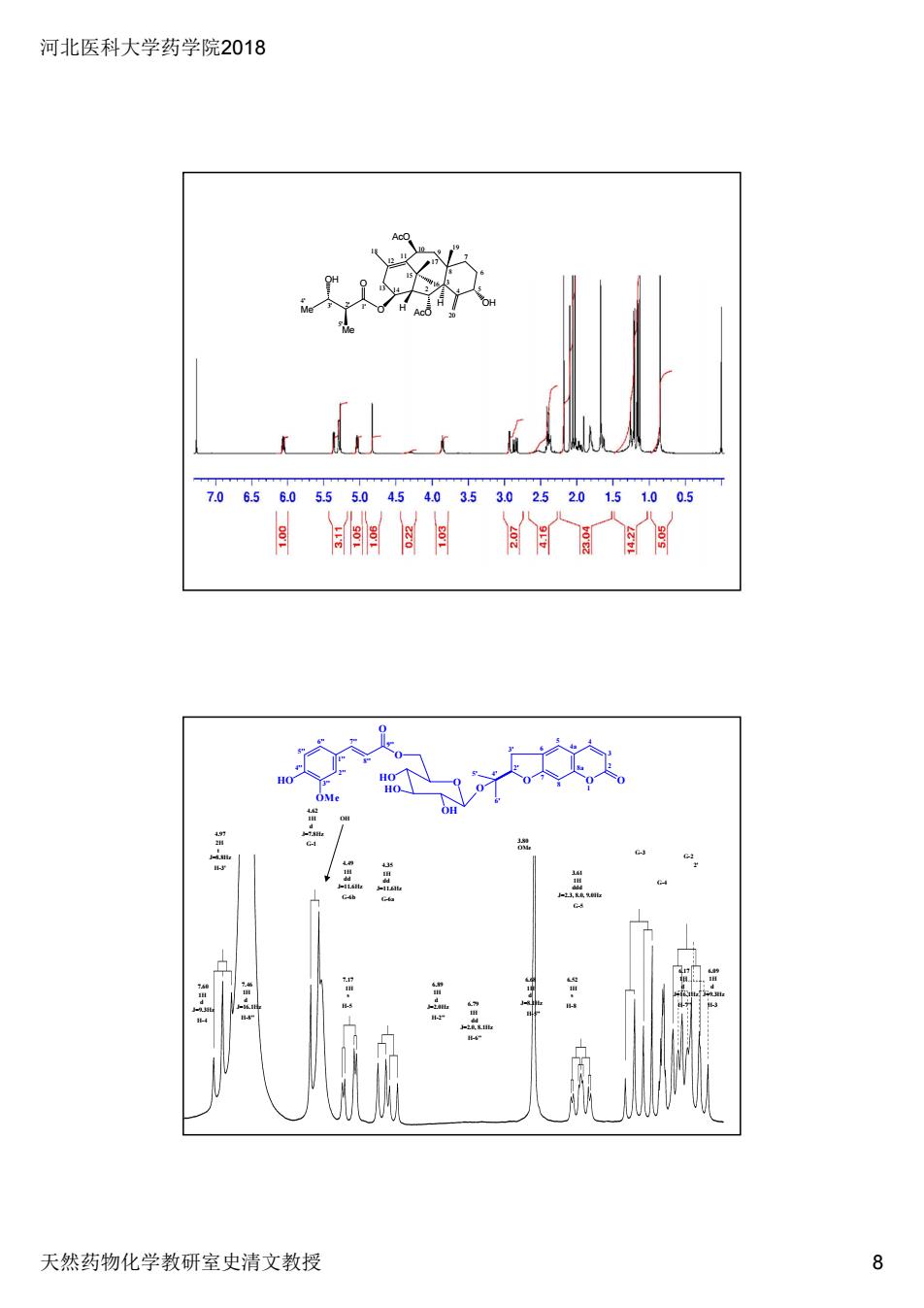
河北医科大学药学院2018 天然药物化学教研室史清文教授 8 H AcO H OH 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 1' O AcO O Me Me OH 3 2' ' 4' 5' 4.97 2H t J=8.8Hz H-3' 4.62 1H d J=7.8Hz G-1 4.49 1H dd J=11.6Hz G-6b 4.35 1H dd J=11.6Hz G-6a OH 3.80 OMe 3.61 1H ddd J=2.3, 8.0, 9.0Hz G-5 G-3 G-4 G-2 2' 7.60 1H d J=9.3Hz H-4 7.46 1H d J=16.1Hz H-8" 7.17 1H s H-5 6.89 1H d J=2.0Hz H-2" 6.79 1H dd J=2.0, 8.1Hz H-6" 6.68 1H d J=8.1Hz H-5" 6.52 1H s H-8 6.17 1H d J=16.1Hz H-7" 6.09 1H d J=9.3Hz H-3 1'' 6'' 5'' 4'' 3'' 2'' 8a 4a 8 7 6 5 4 3 2 1 2' 3' 5' 4' 6' O O O O OH HO HO O O O HO OMe 7'' 8'' 9
河北医科大学药学院2018 TheH NMR Spectrum of Tetraacetyl6O-(trans-Feruloyl)Nodakenin] The H NMR Spectrum of Tetraacetyl[6'-O-(trans-Feruloyl)nodakenin] 天然药物化学教研室史清文教授
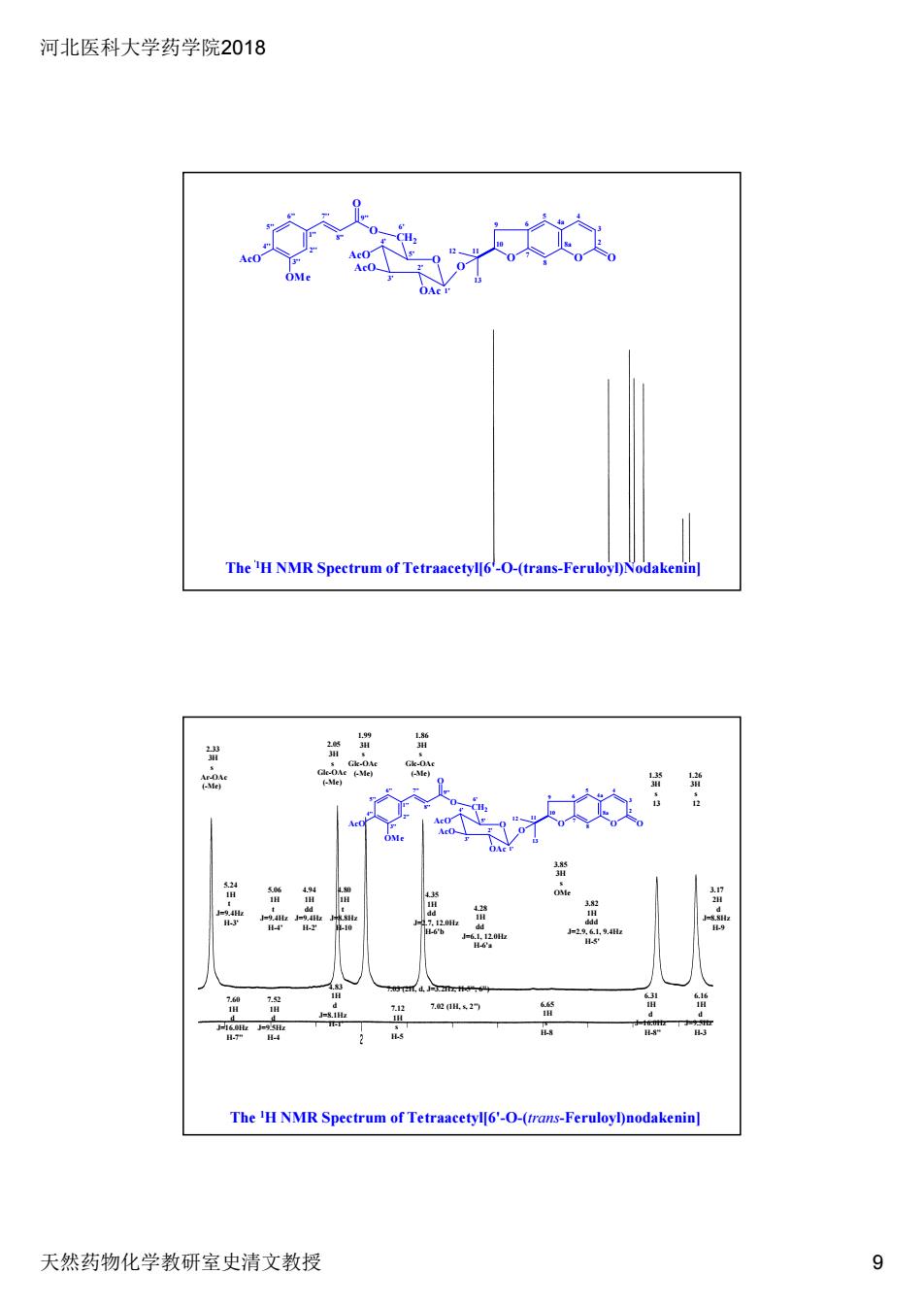
河北医科大学药学院2018 天然药物化学教研室史清文教授 9 The 1H NMR Spectrum of Tetraacetyl[6'-O-(trans-Feruloyl)Nodakenin] 1'' 6'' 5'' 4'' 3'' 2'' 8a 4a 8 7 6 5 4 3 2 10 9 12 11 13 O O O O OAc AcO AcO O CH2 OMe AcO O O 7'' 8'' 9'' 6' 1' 2' 3' 4' 5' 7.60 1H d J=16.0Hz H-7" 7.52 1H d J=9.5Hz H-4 7.12 1H s H-5 7.03 (2H, d, J=3.2Hz, H-5", 6") 7.02 (1H, s, 2") 6.65 1H s H-8 6.31 1H d J=16.0Hz H-8" 6.16 1H d J=9.5Hz H-3 5.24 1H t J=9.4Hz H-3' 5.06 1H t J=9.4Hz H-4' 4.94 1H dd J=9.4Hz H-2' 4.80 1H t J=8.8Hz H-10 4.83 1H d J=8.1Hz H-1' 4.35 1H dd J=2.7, 12.0Hz H-6'b 4.28 1H dd J=6.1, 12.0Hz H-6'a 3.82 1H ddd J=2.9, 6.1, 9.4Hz H-5' 3.85 3H s OMe 3.17 2H d J=8.8Hz H-9 2.33 3H s Ar-OAc (-Me) 2.05 3H s Glc-OAc (-Me) 1.99 3H s Glc-OAc (-Me) 1.86 3H s Glc-OAc (-Me) 1.35 3H s 13 1.26 3H s 1'' 12 6'' 5'' 4'' 3'' 2'' 8a 4a 8 7 6 5 4 3 2 10 9 12 11 13 O O O O OAc AcO AcO O CH2 OMe AcO O O 7'' 8'' 9'' 6' 1' 2' 3' 4' 5' The 1H NMR Spectrum of Tetraacetyl[6'-O-(trans-Feruloyl)nodakenin]
河北医科大学药学院2018 m06004030200 0160140"120T10080"60"40"20m Chemical Shift (pom) 天然药物化学教研室史清文教授 10
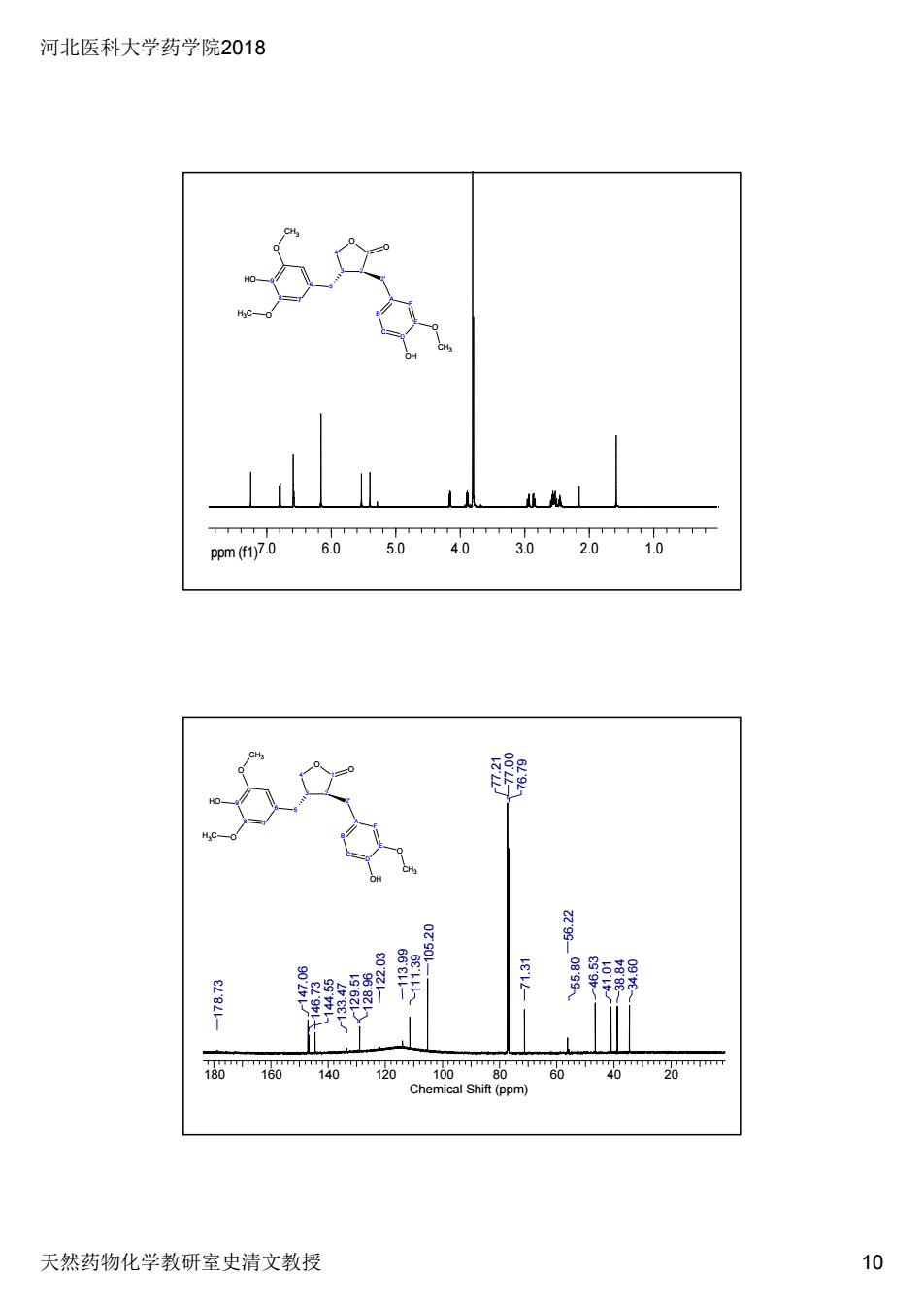
河北医科大学药学院2018 天然药物化学教研室史清文教授 10 ppm (f1)7.0 6.0 5.0 4.0 3.0 2.0 1.0 4 O 3 1 2 O 5 6 7 8 9 O HO O CH3 H3C 2' A F B E C D OH O CH3 4 O 3 1 2 O 5 6 7 8 9 O HO O CH3 H3C 2' A F B E C D OH O CH3 180 160 140 120 100 80 60 40 20 Chemical Shift (ppm) 55.80 46.53 41.01 38.84 34.60 56.22 71.31 76.79 77.21 77.00 105.20 111.39 122.03 113.99 129.51 128.96 133.47 146.73 144.55 147.06 178.73