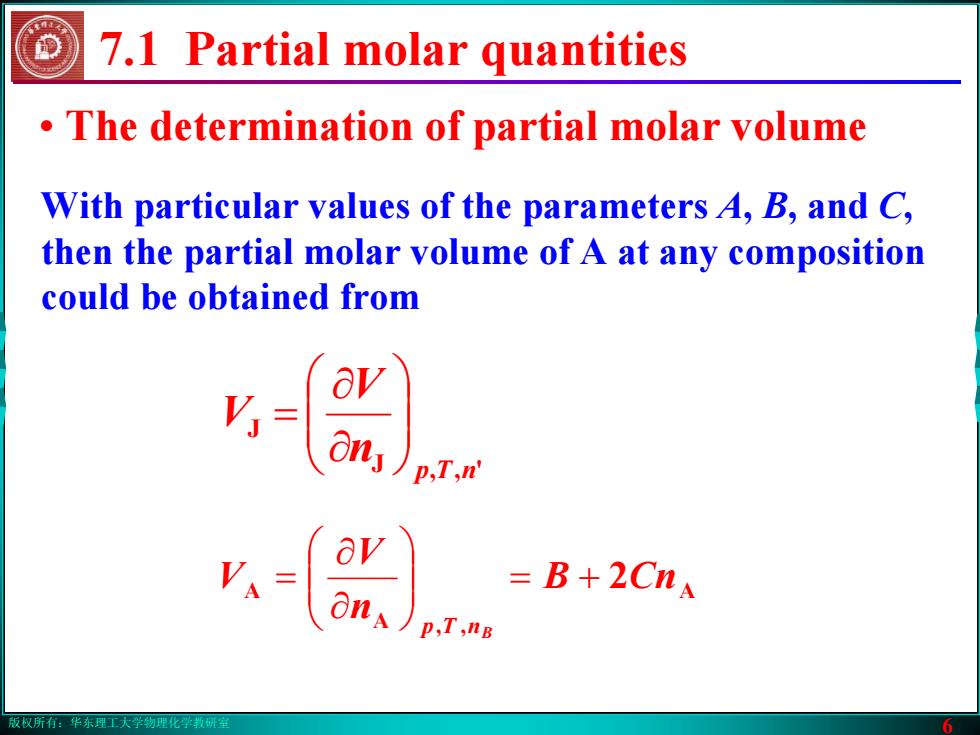
版权所有:华东理工大学物理化学教研室 6 • The determination of partial molar volume 7.1 Partial molar quantities With particular values of the parameters A, B, and C, then the partial molar volume of A at any composition could be obtained from A A , A 2CnB n V V nTp B += ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ = J ', J nTp n V V ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ =
版权所有:华东理工大学物理化学教研室 6 • The determination of partial molar volume 7.1 Partial molar quantities With particular values of the parameters A, B, and C, then the partial molar volume of A at any composition could be obtained from A A , A 2CnB n V V nTp B += ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ = J ', J nTp n V V ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ =
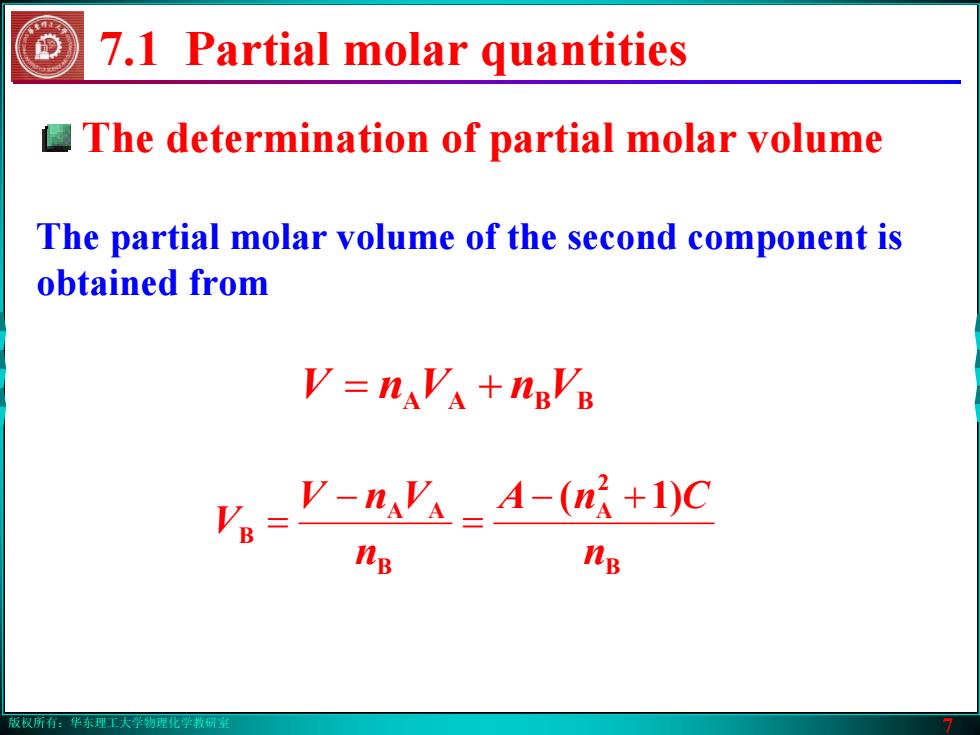
版权所有:华东理工大学物理化学教研室 7 The determination of partial molar volume 7.1 Partial molar quantities B 2 A B AA B )1( n CnA n VnV V +− = − = The partial molar volume of the second component is obtained from = + VnVnV BBAA
版权所有:华东理工大学物理化学教研室 7 The determination of partial molar volume 7.1 Partial molar quantities B 2 A B AA B )1( n CnA n VnV V +− = − = The partial molar volume of the second component is obtained from = + VnVnV BBAA
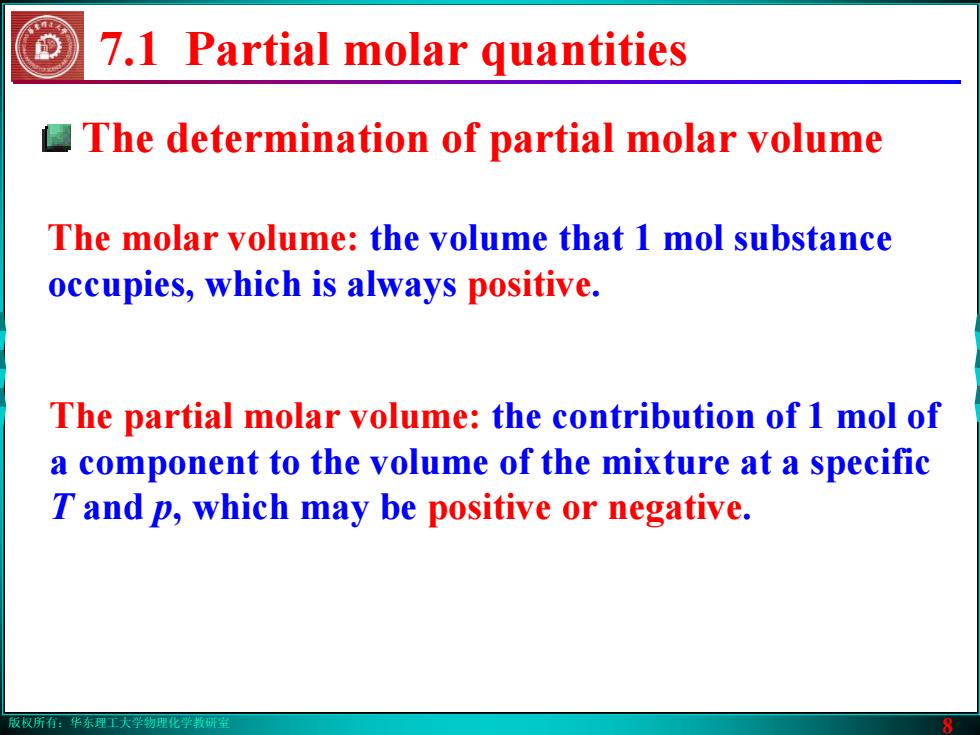
版权所有:华东理工大学物理化学教研室 8 7.1 Partial molar quantities The molar volume: the volume that 1 mol substance occupies, which is always positive. The partial molar volume: the contribution of 1 mol of a component to the volume of the mixture at a specific T and p, which may be positive or negative. The determination of partial molar volume
版权所有:华东理工大学物理化学教研室 8 7.1 Partial molar quantities The molar volume: the volume that 1 mol substance occupies, which is always positive. The partial molar volume: the contribution of 1 mol of a component to the volume of the mixture at a specific T and p, which may be positive or negative. The determination of partial molar volume
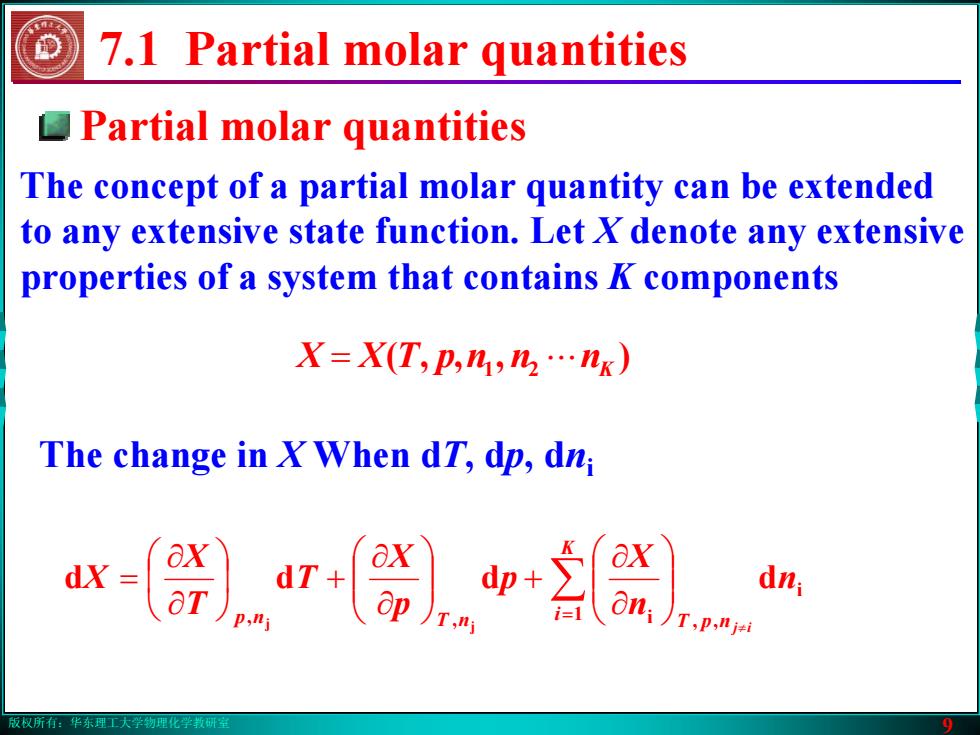
版权所有:华东理工大学物理化学教研室 9 Partial molar quantities The concept of a partial molar quantity can be extended to any extensive state function. Let X denote any extensive properties of a system that contains K components 7.1 Partial molar quantities The change in X When dT, dp, dni ) ,( 21 nnnpTXX K = ⋅⋅⋅ ∑ = ≠ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ + ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ ⎟ + ⎠⎞ ⎜⎝⎛ ∂∂ = Ki np nT npT n nX p pX T TX X ij 1 i , i , , d d d d j j
版权所有:华东理工大学物理化学教研室 9 Partial molar quantities The concept of a partial molar quantity can be extended to any extensive state function. Let X denote any extensive properties of a system that contains K components 7.1 Partial molar quantities The change in X When dT, dp, dni ) ,( 21 nnnpTXX K = ⋅⋅⋅ ∑ = ≠ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ + ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ ⎟ + ⎠⎞ ⎜⎝⎛ ∂∂ = Ki np nT npT n nX p pX T TX X ij 1 i , i , , d d d d j j
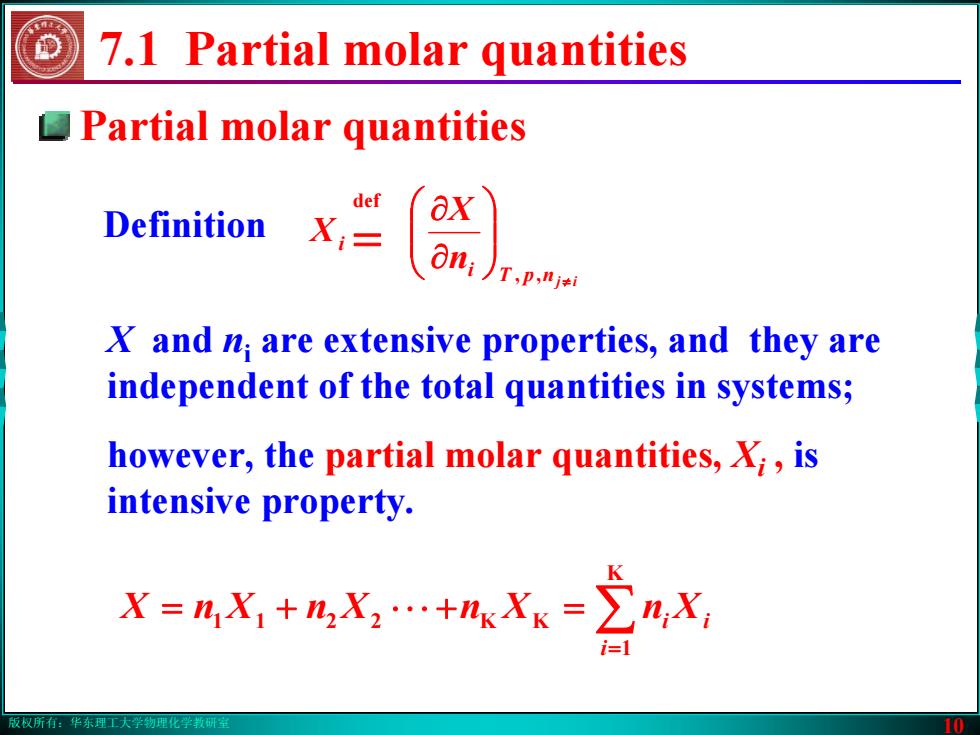
版权所有:华东理工大学物理化学教研室 10 7.1 Partial molar quantities Definition npT ij i i n X X ≠ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ = , def X and ni are extensive properties, and they are independent of the total quantities in systems; however, the partial molar quantities, Xi , is intensive property. ∑ = =+⋅⋅⋅+= K 1 2211 KK i XnXnXnXnX ii Partial molar quantities
版权所有:华东理工大学物理化学教研室 10 7.1 Partial molar quantities Definition npT ij i i n X X ≠ ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ = , def X and ni are extensive properties, and they are independent of the total quantities in systems; however, the partial molar quantities, Xi , is intensive property. ∑ = =+⋅⋅⋅+= K 1 2211 KK i XnXnXnXnX ii Partial molar quantities