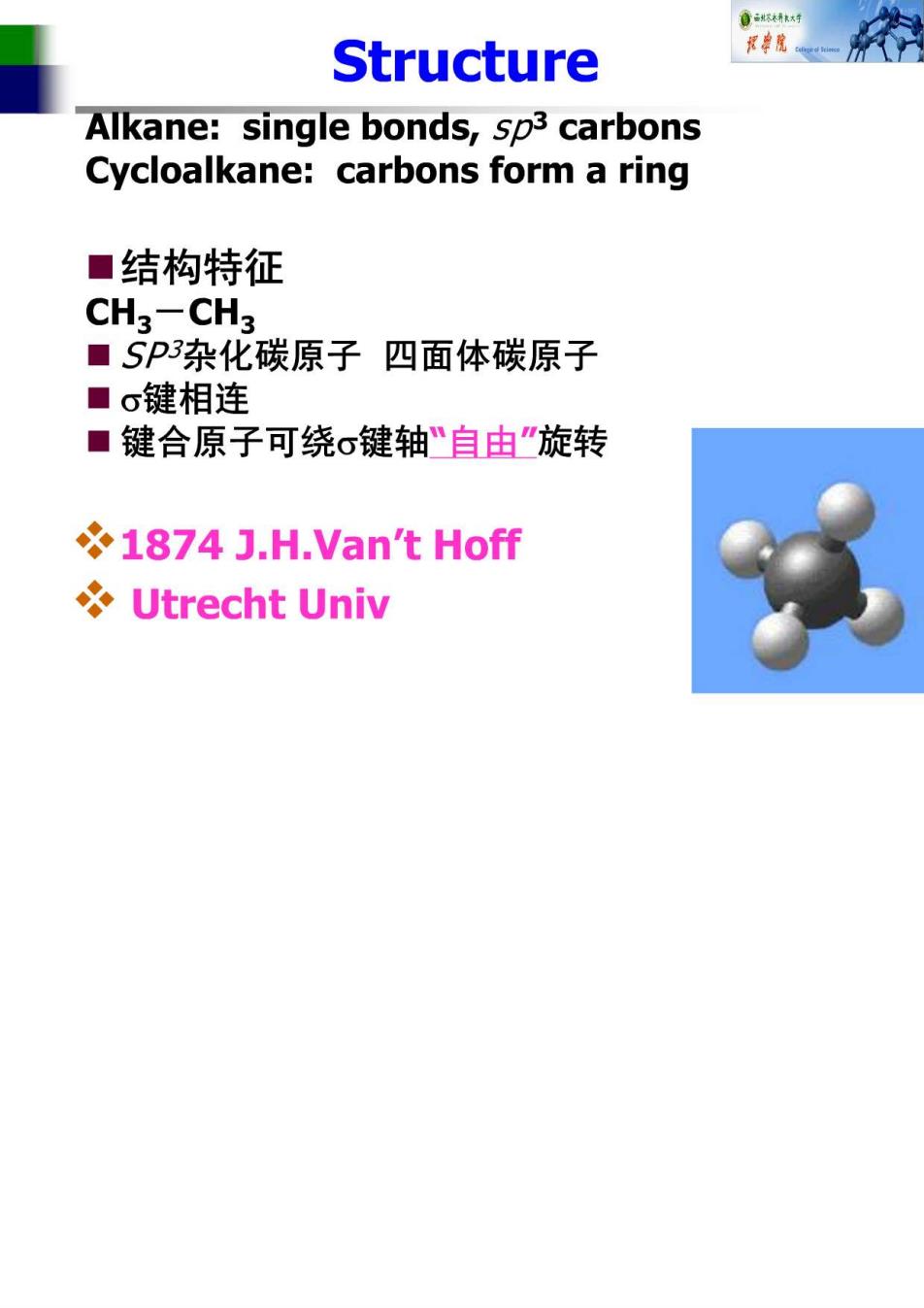
Structure Alkane:single bonds,sp3 carbons Cycloalkane:carbons form a ring ■结构特征 CH3-CH3 ■SP杂化碳原子四面体碳原子 ■o键相连 ■键合原子可绕σ键轴“自由“旋转 1874 J.H.Van't Hoff Utrecht Univ

Reactivity of alkanes Chemical stability:C-H;C-C ■不与强酸、强碱、氧化剂反应 ■自由基反应hv
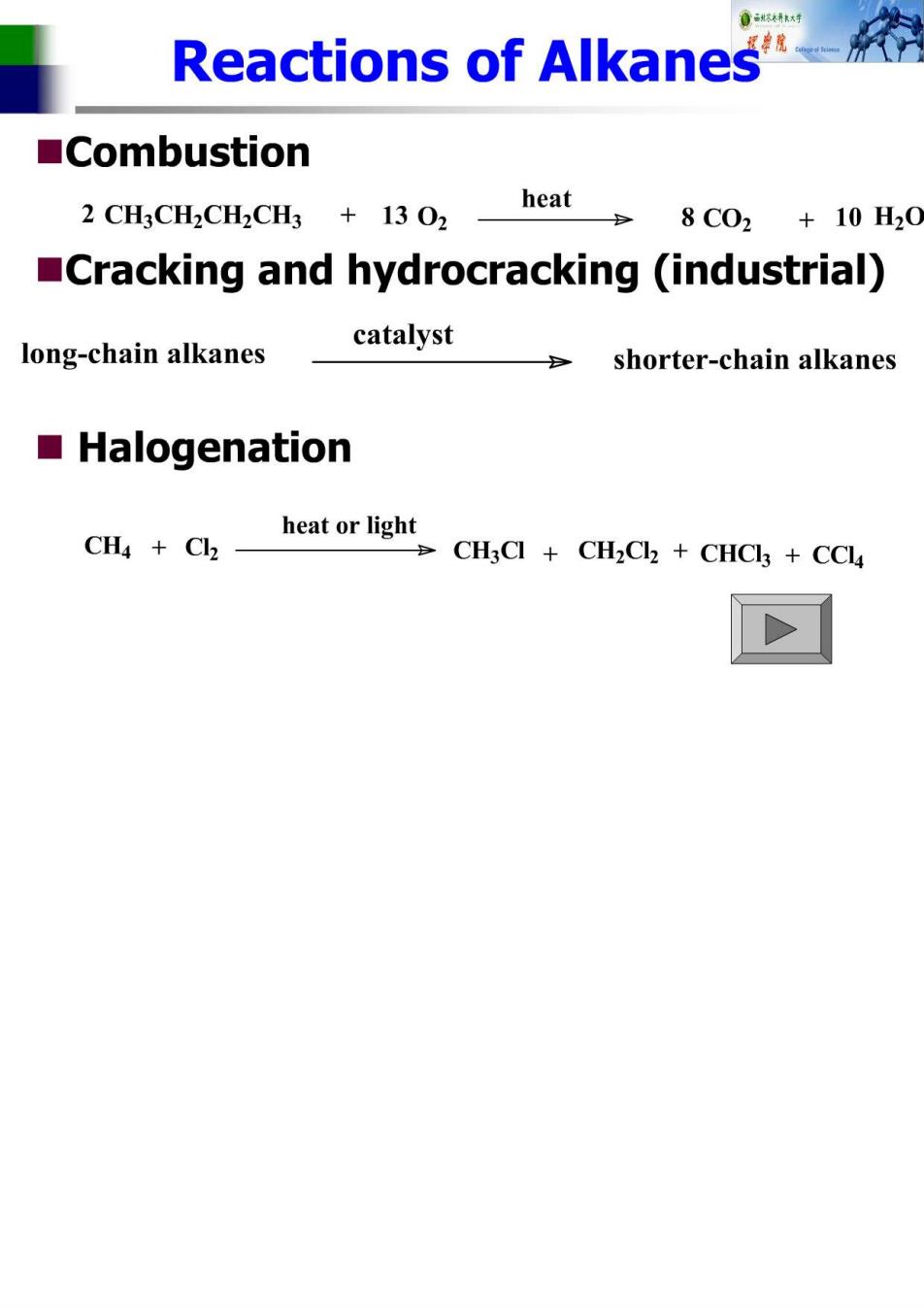
不 Reactions of Alkanes ■Combustion 2 CH3CH2CH2CH3 +13 02 heat 8C02+10H20 Cracking and hydrocracking (industrial) catalyst long-chain alkanes -shorter-chain alkanes ■Halogenation CH Cla heat or light CH3Cl CH2Cl2 CHCl3 CCl4
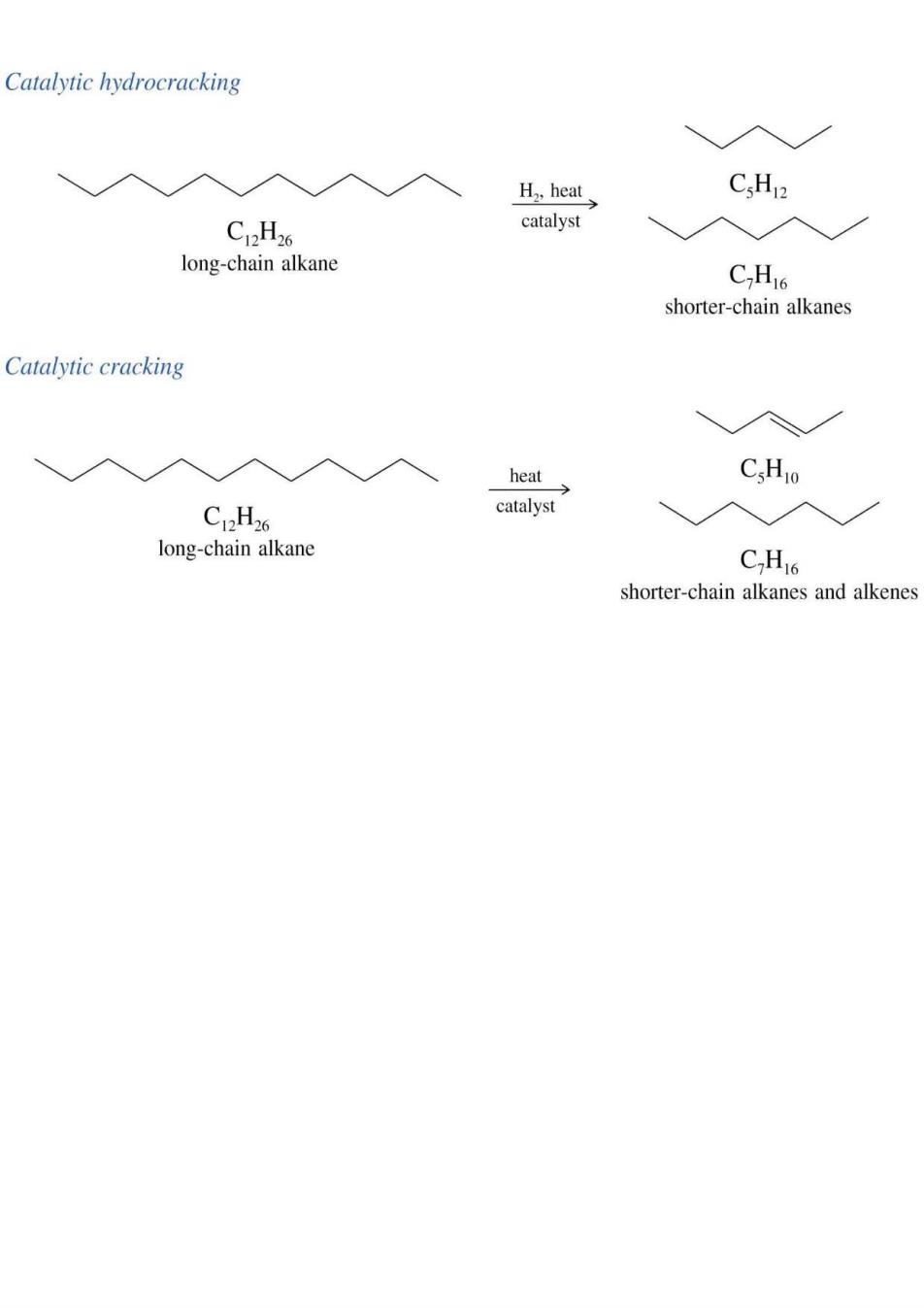
Catalytic hydrocracking H.,heat C.H2 C2H6 catalyst 入入 long-chain alkane C.H16 shorter-chain alkanes Catalytic cracking heat CsHIo C12H6 catalyst long-chain alkane CHI6 shorter-chain alkanes and alkenes

华就 Petroleum Refining ■Cracking >converts high molecular weight hydrocarbons to more useful,low molecular weight ones ■Reforming >increases branching of hydrocarbon chains >branched hydrocarbons have better burning characteristics for automobile engines